Two-Photon Polymerization: Fundamentals, Materials, and Chemical Modification Strategies
- PMID: 36585380
- PMCID: PMC9982557
- DOI: 10.1002/advs.202204072
Two-Photon Polymerization: Fundamentals, Materials, and Chemical Modification Strategies
Abstract
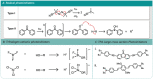
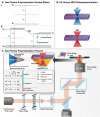
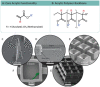
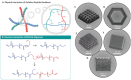
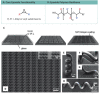
Two-photon polymerization (TPP) has become a premier state-of-the-art method for microscale fabrication of bespoke polymeric devices and surfaces. With applications ranging from the production of optical, drug delivery, tissue engineering, and microfluidic devices, TPP has grown immensely in the past two decades. Significantly, the field has expanded from standard acrylate- and epoxy-based photoresists to custom formulated monomers designed to change the hydrophilicity, surface chemistry, mechanical properties, and more of the resulting structures. This review explains the essentials of TPP, from its initial conception through to standard operating principles and advanced chemical modification strategies for TPP materials. At the outset, the fundamental chemistries of radical and cationic polymerization are described, along with strategies used to tailor mechanical and functional properties. This review then describes TPP systems and introduces an array of commonly used photoresists including hard polyacrylic resins, soft hydrogel acrylic esters, epoxides, and organic/inorganic hybrid materials. Specific examples of each class-including chemically modified photoresists-are described to inform the understanding of their applications to the fields of tissue-engineering scaffolds, micromedical, optical, and drug delivery devices.
Keywords: 3D laser printing; direct laser writing; modification strategies; photoresists; two-photon polymerization.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Nesvadba P., in Encyclopedia of Radicals in Chemistry, Biology and Materials (Eds: Chatgilialoglu C., Studer A.), Wiley, Chichester, UK: 2012.
-
- Destarac M., Polym. Chem. 2018, 9, 4947.
-
- Hawker C. J., J. Am. Chem. Soc. 1994, 116, 11185.
-
- Hawker C. J., Bosman A. W., Harth E., Chem. Rev. 2001, 101, 3661. - PubMed
-
- Grubbs R. B., Grubbs R. H., Macromolecules 2017, 50, 6979.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
