Parabrachial-to-parasubthalamic nucleus pathway mediates fear-induced suppression of feeding in male mice
- PMID: 36585411
- PMCID: PMC9803671
- DOI: 10.1038/s41467-022-35634-2
Parabrachial-to-parasubthalamic nucleus pathway mediates fear-induced suppression of feeding in male mice
Abstract
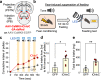
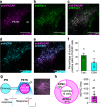
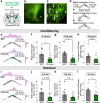
Feeding behavior is adaptively regulated by external and internal environment, such that feeding is suppressed when animals experience pain, sickness, or fear. While the lateral parabrachial nucleus (lPB) plays key roles in nociception and stress, neuronal pathways involved in feeding suppression induced by fear are not fully explored. Here, we investigate the parasubthalamic nucleus (PSTN), located in the lateral hypothalamus and critically involved in feeding behaviors, as a target of lPB projection neurons. Optogenetic activation of lPB-PSTN terminals in male mice promote avoidance behaviors, aversive learning, and suppressed feeding. Inactivation of the PSTN and lPB-PSTN pathway reduces fear-induced feeding suppression. Activation of PSTN neurons expressing pituitary adenylate cyclase-activating polypeptide (PACAP), a neuropeptide enriched in the PSTN, is sufficient for inducing avoidance behaviors and feeding suppression. Blockade of PACAP receptors impaires aversive learning induced by lPB-PSTN photomanipulation. These findings indicate that lPB-PSTN pathway plays a pivotal role in fear-induced feeding suppression.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Stimulation of lateral parabrachial (LPB) to central amygdala (CeA) pituitary adenylate cyclase-activating polypeptide (PACAP) neurons induces anxiety-like behavior and mechanical allodynia.Pharmacol Biochem Behav. 2023 Sep;230:173605. doi: 10.1016/j.pbb.2023.173605. Epub 2023 Jul 25. Pharmacol Biochem Behav. 2023. PMID: 37499765
-
At the heart of the interoception network: Influence of the parasubthalamic nucleus on autonomic functions and motivated behaviors.Neuropharmacology. 2022 Feb 15;204:108906. doi: 10.1016/j.neuropharm.2021.108906. Epub 2021 Nov 29. Neuropharmacology. 2022. PMID: 34856204 Free PMC article. Review.
-
Parabrachial Pituitary Adenylate Cyclase-Activating Polypeptide Activation of Amygdala Endosomal Extracellular Signal-Regulated Kinase Signaling Regulates the Emotional Component of Pain.Biol Psychiatry. 2017 Apr 15;81(8):671-682. doi: 10.1016/j.biopsych.2016.08.025. Epub 2016 Aug 29. Biol Psychiatry. 2017. PMID: 28057459 Free PMC article.
-
A discrete parasubthalamic nucleus subpopulation plays a critical role in appetite suppression.Elife. 2022 May 4;11:e75470. doi: 10.7554/eLife.75470. Elife. 2022. PMID: 35507386 Free PMC article.
-
The parasubthalamic nucleus: A novel eating center in the brain.Prog Neuropsychopharmacol Biol Psychiatry. 2025 Jan 10;136:111250. doi: 10.1016/j.pnpbp.2025.111250. Epub 2025 Jan 7. Prog Neuropsychopharmacol Biol Psychiatry. 2025. PMID: 39788409 Review.
Cited by
-
A top-down insular cortex circuit crucial for non-nociceptive fear learning.Sci Adv. 2025 May 9;11(19):eadt6996. doi: 10.1126/sciadv.adt6996. Epub 2025 May 9. Sci Adv. 2025. PMID: 40344067 Free PMC article.
-
All-optical presynaptic plasticity induction by photoactivated adenylyl cyclase targeted to axon terminals.Cell Rep Methods. 2024 Apr 22;4(4):100740. doi: 10.1016/j.crmeth.2024.100740. Epub 2024 Mar 22. Cell Rep Methods. 2024. PMID: 38521059 Free PMC article.
-
The functional heterogeneity of PACAP: Stress, learning, and pathology.Neurobiol Learn Mem. 2023 Sep;203:107792. doi: 10.1016/j.nlm.2023.107792. Epub 2023 Jun 25. Neurobiol Learn Mem. 2023. PMID: 37369343 Free PMC article. Review.
-
Hypothalamic-hindbrain circuit for consumption-induced fear regulation.Nat Commun. 2024 Sep 4;15(1):7728. doi: 10.1038/s41467-024-51983-6. Nat Commun. 2024. PMID: 39231981 Free PMC article.
-
Whole-Brain Monosynaptic Inputs to Parasubthalamic Calretinin Neurons in Mice.Mol Neurobiol. 2025 May 30. doi: 10.1007/s12035-025-05087-x. Online ahead of print. Mol Neurobiol. 2025. PMID: 40445482
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

