Time-dose reciprocity mechanism for the inactivation of Escherichia coli explained by a stochastic process with two inactivation effects
- PMID: 36585428
- PMCID: PMC9801147
- DOI: 10.1038/s41598-022-26783-x
Time-dose reciprocity mechanism for the inactivation of Escherichia coli explained by a stochastic process with two inactivation effects
Abstract
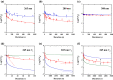
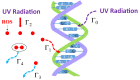
There is a great demand for developing and demonstrating novel disinfection technologies for protection against various pathogenic viruses and bacteria. In this context, ultraviolet (UV) irradiation offers an effective and convenient method for the inactivation of pathogenic microorganisms. The quantitative evaluation of the efficacy of UV sterilization relies on the simple time-dose reciprocity law proposed by Bunsen-Roscoe. However, the inactivation rate constants reported in the literature vary widely, even at the same dose and wavelength of irradiation. Thus, it is likely that the physical mechanism of UV inactivation cannot be described by the simple time-dose reciprocity law but requires a secondary inactivation process, which must be identified to clarify the scientific basis. In this paper, we conducted a UV inactivation experiment with Escherichia coli at the same dose but with different irradiances and irradiation durations, varying the irradiance by two to three orders of magnitude. We showed that the efficacy of inactivation obtained by UV-light emitting diode irradiation differs significantly by one order of magnitude at the same dose but different irradiances at a fixed wavelength. To explain this, we constructed a stochastic model introducing a second inactivation rate, such as that due to reactive oxygen species (ROS) that contribute to DNA and/or protein damage, together with the fluence-based UV inactivation rate. By solving the differential equations based on this model, the efficacy of inactivation as a function of the irradiance and irradiation duration under the same UV dose conditions was clearly elucidated. The proposed model clearly shows that at least two inactivation rates are involved in UV inactivation, where the generally used UV inactivation rate does not depend on the irradiance, but the inactivation rate due to ROS does depend on the irradiance. We conclude that the UV inactivation results obtained to date were simply fitted by one inactivation rate that superimposed these two inactivation rates. The effectiveness of long-term UV irradiation at a low irradiance but the same dose provides useful information for future disinfection technologies such as the disinfection of large spaces, for example, hospital rooms using UV light, because it can reduce the radiation dose and its risk to the human body.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

