A phase III, multicenter, randomized controlled trial of preoperative versus postoperative stereotactic radiosurgery for patients with surgically resectable brain metastases
- PMID: 36585629
- PMCID: PMC9805276
- DOI: 10.1186/s12885-022-10480-z
A phase III, multicenter, randomized controlled trial of preoperative versus postoperative stereotactic radiosurgery for patients with surgically resectable brain metastases
Abstract
Background: Postoperative stereotactic radiosurgery (SRS) is a standard management option for patients with resected brain metastases. Preoperative SRS may have certain advantages compared to postoperative SRS, including less uncertainty in delineation of the intact tumor compared to the postoperative resection cavity, reduced rate of leptomeningeal dissemination postoperatively, and a lower risk of radiation necrosis. The recently published ASCO-SNO-ASTRO consensus statement provides no recommendation for the preferred sequencing of radiotherapy and surgery for patients receiving both treatments for their brain metastases.
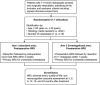
Methods: This multicenter, randomized controlled trial aims to recruit 88 patients with resectable brain metastases over an estimated three-year period. Patients with ten or fewer brain metastases with at least one resectable, fulfilling inclusion criteria will be randomized to postoperative SRS (standard arm) or preoperative SRS (investigational arm) in a 1:1 ratio. Randomization will be stratified by age (< 60 versus ≥60 years), histology (melanoma/renal cell carcinoma/sarcoma versus other), and number of metastases (one versus 2-10). In the standard arm, postoperative SRS will be delivered within 3 weeks of surgery, and all unresected metastases will receive primary SRS. In the investigational arm, enrolled patients will receive SRS of all brain metastases followed by surgery of resectable metastases within one week of SRS. In either arm, single fraction or hypofractionated SRS in three or five fractions is permitted. The primary endpoint is to assess local control at 12 months in both arms. Secondary endpoints include local control at other time points, regional/distant brain recurrence rates, leptomeningeal recurrence rates, overall survival, neurocognitive outcomes, and adverse radiation events including radiation necrosis rates in both arms.
Discussion: This trial addresses the unanswered question of the optimal sequencing of surgery and SRS in the management of patients with resectable brain metastases. No randomized data comparing preoperative and postoperative SRS for patients with brain metastases has been published to date.
Trial registration: Clinicaltrials.gov , NCT04474925; registered on July 17, 2020. Protocol version 1.0 (January 31, 2020).
Sponsor: Alberta Health Services, Edmonton, Canada (Samir Patel, MD).
Keywords: Brain metastases; Neurocognitive outcomes; Postoperative; Preoperative; Quality of life; Stereotactic radiosurgery.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
References
-
- National Comprehensive Cancer Network. Central nervous system cancers Version 01.2022. Available at http://www.nccn.org/professionals/physician_gls/pdf/cns.pdf.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical