Phosphatidylserine-mediated oral tolerance
- PMID: 36586393
- PMCID: PMC11034824
- DOI: 10.1016/j.cellimm.2022.104660
Phosphatidylserine-mediated oral tolerance
Abstract
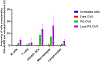
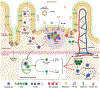
Phosphatidylserine (PS) is an anionic phospholipid exposed on the surface of apoptotic cells. The exposure of PS typically recruits and signals phagocytes to engulf and silently clear these dying cells to maintain tolerance via immunological ignorance. However, recent and emerging evidence has demonstrated that PS converts an "immunogen" into a "tolerogen", and PS exposure on the surface of cells or vesicles actively promotes a tolerogenic environment. This tolerogenic property depends on the biophysical characteristics of PS-containing vesicles, including PS density on the particle surface to effectively engage tolerogenic receptors, such as TIM-4, which is exclusively expressed on the surface of antigen-presenting cells. We harnessed the cellular and molecular mechanistic insight of PS-mediated immune regulation to design an effective oral tolerance approach. This immunotherapy has been shown to prevent/reduce immune response against life-saving protein-based therapies, food allergens, autoantigens, and the antigenic viral capsid peptide commonly used in gene therapy, suggesting a broad spectrum of potential clinical applications. Given the good safety profile of PS together with the ease of administration, oral tolerance achieved with PS-based nanoparticles has a very promising therapeutic impact.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
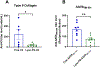
References
-
- Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, Campbell PA, Henson PM. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992; 149: 4029–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

