Comparison of model predictions of typhoid conjugate vaccine public health impact and cost-effectiveness
- PMID: 36586741
- PMCID: PMC9880559
- DOI: 10.1016/j.vaccine.2022.12.032
Comparison of model predictions of typhoid conjugate vaccine public health impact and cost-effectiveness
Abstract
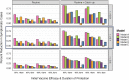
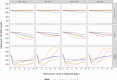
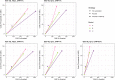
Models are useful to inform policy decisions on typhoid conjugate vaccine (TCV) deployment in endemic settings. However, methodological choices can influence model-predicted outcomes. To provide robust estimates for the potential public health impact of TCVs that account for structural model differences, we compared four dynamic and one static mathematical model of typhoid transmission and vaccine impact. All models were fitted to a common dataset of age-specific typhoid fever cases in Kolkata, India. We evaluated three TCV strategies: no vaccination, routine vaccination at 9 months of age, and routine vaccination at 9 months with a one-time catch-up campaign (ages 9 months to 15 years). The primary outcome was the predicted percent reduction in symptomatic typhoid cases over 10 years after vaccine introduction. For three models with economic analyses (Models A-C), we also compared the incremental cost-effectiveness ratios (ICERs), calculated as the incremental cost (US$) per disability-adjusted life-year (DALY) averted. Routine vaccination was predicted to reduce symptomatic cases by 10-46 % over a 10-year time horizon under an optimistic scenario (95 % initial vaccine efficacy and 19-year mean duration of protection), and by 2-16 % under a pessimistic scenario (82 % initial efficacy and 6-year mean protection). Adding a catch-up campaign predicted a reduction in incidence of 36-90 % and 6-35 % in the optimistic and pessimistic scenarios, respectively. Vaccine impact was predicted to decrease as the relative contribution of chronic carriers to transmission increased. Models A-C all predicted routine vaccination with or without a catch-up campaign to be cost-effective compared to no vaccination, with ICERs varying from $95-789 per DALY averted; two models predicted the ICER of routine vaccination alone to be greater than with the addition of catch-up campaign. Despite differences in model-predicted vaccine impact and cost-effectiveness, routine vaccination plus a catch-up campaign is likely to be impactful and cost-effective in high incidence settings such as Kolkata.
Keywords: Economic evaluation; Mathematical modeling; Model comparison; Typhoid conjugate vaccines; Typhoid fever.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: VEP is a member of the WHO Immunization and Vaccine-related Implementation Research Advisory Committee (IVIR-AC). All other authors have no conflicts to declare.
Figures

References
-
- Stanaway J.D., Reiner R.C., Blacker B.F., Goldberg E.M., Khalil I.A., Troeger C.E., et al. The global burden of typhoid and paratyphoid fevers: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis. 2019;19:369–381. doi: 10.1016/S1473-3099(18)30685-6. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

