Immune cell populations differ in patients undergoing revision total knee arthroplasty for arthrofibrosis
- PMID: 36587032
- PMCID: PMC9805429
- DOI: 10.1038/s41598-022-22175-3
Immune cell populations differ in patients undergoing revision total knee arthroplasty for arthrofibrosis
Abstract
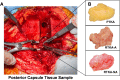
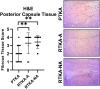
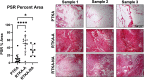
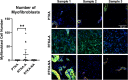
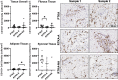
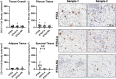
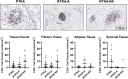
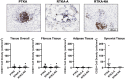
Arthrofibrosis following total knee arthroplasty (TKA) is a debilitating condition typically diagnosed based on clinical findings. To gain insight into the histopathologic immune cell microenvironment of arthrofibrosis, we assessed the extent of tissue fibrosis and quantified immune cell populations in specific tissue regions of the posterior capsule. We investigated specimens from three prospectively-collected, matched cohorts, grouped as patients receiving a primary TKA for osteoarthritis, revision TKA for arthrofibrosis, and revision TKA for non-arthrofibrotic, non-infectious reasons. Specimens were evaluated using hematoxylin and eosin staining, picrosirius red staining, immunofluorescence, and immunohistochemistry with Aperio®-based digital image analysis. Increased collagen deposition and increased number of α-SMA/ACTA2 expressing myofibroblasts were present in the arthrofibrosis group compared to the two non-arthrofibrotic groups. CD163 + macrophages were the most abundant immune cell type in any capsular sample with specific enrichment in the synovial tissue. CD163 + macrophages were significantly decreased in the fibrotic tissue region of arthrofibrosis patients compared to the patients with primary TKA, and significantly increased in adipose tissue region of arthrofibrotic specimens compared to non-arthrofibrotic specimens. Synovial CD117 + mast cells were significantly decreased in arthrofibrotic adipose tissue. Together, these findings inform diagnostic and targeted therapeutic strategies by providing insight into the underlying pathogenetic mechanisms of arthrofibrosis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Gwam CU, et al. Use of neuromuscular electrical stimulation during physical therapy may reduce the incidence of arthrofibrosis after total knee arthroplasty. Surg. Technol. Int. 2018;32:356–360. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

