Secondary sclerosing cholangitis and IgG4-sclerosing cholangitis - A review of cholangiographic and ultrasound imaging
- PMID: 36588352
- PMCID: PMC10237613
- DOI: 10.4103/EUS-D-22-00208
Secondary sclerosing cholangitis and IgG4-sclerosing cholangitis - A review of cholangiographic and ultrasound imaging
Abstract
Sclerosing cholangitis (SC) represents a spectrum of chronic progressive cholestatic diseases of the intrahepatic and/or extrahepatic biliary system characterized by patchy inflammation, fibrosis, and stricturing. Primary and secondary SC must be distinguished given the different treatment modalities, risks of malignancy, and progression to portal hypertension, cirrhosis, and hepatic failure. This review focuses on secondary SC and the pathogenic mechanisms, risk factors, clinical presentation, and novel imaging modalities that help to distinguish between these conditions. We explore the detailed use of cholangiography and ultrasound imaging techniques.
Keywords: Cholangiography; IgG4-related sclerosing cholangitis; secondary cholangitis-critically Ill patients; secondary sclerosing cholangitis; ultrasound.
Copyright: © 2022 SCHOLAR MEDIA PUBLISHING.
Conflict of interest statement
Siyu Sun is the Editor-in-Chief of the journal, Christoph F. Dietrich is a Co-Editor-in-Chief, Christian Jenssen and Michael Hocke are Editorial Board Members. This article was subject to the journal’s standard procedures, with peer review handled independently of the editors and their research groups.
Figures

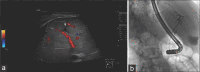

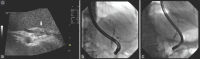


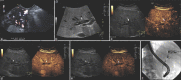
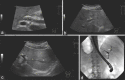
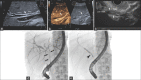
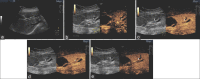
References
-
- Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver –Update 2012:A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187–210. - PubMed
-
- Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver –Update 2012:A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med. 2013;34:11–29. - PubMed
-
- Dietrich CF, Nolsøe CP, Barr RG, et al. Guidelines and good clinical practice recommendations for contrast-enhanced ultrasound (CEUS) in the liver-update 2020 WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol. 2020;46:2579–604. - PubMed
-
- Dietrich CF, Nolsøe CP, Barr RG, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver –Update 2020 –WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultraschall Med. 2020;41:562–85. - PubMed

