Fabrication, assessment, and optimization of alendronate sodium nanoemulsion-based injectable in-situ gel formulation for management of osteoporosis
- PMID: 36588399
- PMCID: PMC9809409
- DOI: 10.1080/10717544.2022.2164094
Fabrication, assessment, and optimization of alendronate sodium nanoemulsion-based injectable in-situ gel formulation for management of osteoporosis
Abstract
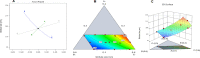
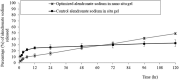
Low bone mass, degeneration of bone tissue, and disruption of bone microarchitecture are all symptoms of the disease osteoporosis, which can decrease bone strength and increase the risk of fractures. The main objective of the current study was to use a phospholipid-based phase separation in-situ gel (PPSG) in combination with an alendronate sodium nanoemulsion (ALS-NE) to help prevent bone resorption in rats. The effect of factors such as concentrations of the ALS aqueous solution, surfactant Plurol Oleique CC 497, and Maisine CC oil on nanoemulsion characteristics such as stability index and globular size was investigated using an l-optimal coordinate exchange statistical design. Injectable PPSG with the best nanoemulsion formulation was tested for viscosity, gel strength, water absorption, and in-vitro ALS release. ALS retention in the rats' muscles was measured after 30 days. The droplet size and stability index of the optimal nanoemulsion were 90 ± 2.0 nm and 85 ± 1.9%, respectively. When mixed with water, the optimal ALS-NE-loaded PPSG became viscous and achieved 36 seconds of gel strength, which was adequate for an injectable in-situ formulation. In comparison with the ALS solution-loaded in-situ gel, the newly created optimal ALS-NE-loaded PPSG produced the sustained and regulated release of ALS; hence, a higher percentage of ALS remained in rats' muscles after 30 days. PPSG that has been loaded with an ALS-NE may therefore be a more auspicious, productive, and effective platform for osteoporosis treatment than conventional oral forms.
Keywords: In situ gel; PPSG; alendronate sodium; design of experiment; nanoemulsion; osteoporosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



References
-
- Abdelbary A, Salem HF, Khallaf RA, et al. (2017). Mucoadhesive niosomal in situ gel for ocular tissue targeting: in vitro and in vivo evaluation of lomefloxacin hydrochloride. Pharm Dev Technol 22:1–9. - PubMed
-
- Alhakamy NA, Hosny KM, Aldryhim AY, et al. (2022). Development and optimization of ofloxacin as solid lipid nanoparticles for enhancement of its ocular activity. J Drug Deliv Sci Technol 72:103373.
-
- Ali SA, Sindi AM, Mair YH, et al. (2021). Oral gel loaded by ethotransfersomes of antifungal drug for oral thrush: preparation, characterization, and assessment of antifungal activity. J Drug Deliv Sci Technol 66:102841.
-
- Alkhalidi HM, Naguib GH, Kurakula M, et al. (2018). In vitro and preclinical assessment of factorial design based nanoethosomal transdermal film formulation of mefenamic acid to overcome barriers to its use in relieving pain and inflammation. J Drug Deliv Sci Technol 48:450–6.
-
- Bauer DC, Black D, Ensrud K, et al. (2000). Upper gastrointestinal tract safety profile of alendronate: the fracture intervention trial. Arch Intern Med 160:517–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
