Pharmacokinetics, distribution, metabolism, and excretion of body-protective compound 157, a potential drug for treating various wounds, in rats and dogs
- PMID: 36588717
- PMCID: PMC9794587
- DOI: 10.3389/fphar.2022.1026182
Pharmacokinetics, distribution, metabolism, and excretion of body-protective compound 157, a potential drug for treating various wounds, in rats and dogs
Abstract
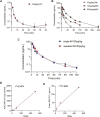
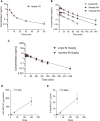
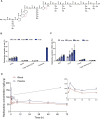
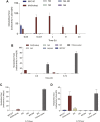
Body-protective compound (BPC) 157 demonstrates protective effects against damage to various organs and tissues. For future clinical applications, we had previously established a solid-phase synthesis process for BPC157, verified its biological activity in different wound models, and completed preclinical safety evaluations. This study aimed to investigate the pharmacokinetics, excretion, metabolism, and distribution profiles of BPC157. After a single intravenous (IV) administration, single intramuscular (IM) administrations at three doses in successive increments along with repeated IM administrations, the elimination half-life (t1/2) of prototype BPC157 was less than 30 min, and BPC157 showed linear pharmacokinetic characteristics in rats and beagle dogs at all doses. The mean absolute bioavailability of BPC157 following IM injection was approximately 14%-19% in rats and 45%-51% in beagle dogs. Using [3H]-labeled BPC157 and radioactivity examination, we proved that the main excretory pathways of BPC157 involved urine and bile. [3H]BPC157 was rapidly metabolized into a variety of small peptide fragments in vivo, thus forming single amino acids that entered normal amino acid metabolism and excretion pathways. In conclusion, this study provides the first analysis of the pharmacokinetics of BPC157, which will be helpful for its translation in the clinic.
Keywords: BPC157; absorption; distribution; excretion; metabolism; pharmacokinetics; wounds.
Copyright © 2022 He, Feng, Guo, Zhou, Li, Zhang, Zhang, Wang, Wang, Hao, Zhang, Gao, Gu, Zhang, Li and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Preclinical safety evaluation of body protective compound-157, a potential drug for treating various wounds.Regul Toxicol Pharmacol. 2020 Jul;114:104665. doi: 10.1016/j.yrtph.2020.104665. Epub 2020 Apr 22. Regul Toxicol Pharmacol. 2020. PMID: 32334036
-
Absorption, distribution, metabolism and excretion of glucosamine sulfate. A review.Arzneimittelforschung. 2001 Sep;51(9):699-725. doi: 10.1055/s-0031-1300105. Arzneimittelforschung. 2001. PMID: 11642003 Review.
-
Pharmacokinetics of 4-acetylaminophenylacetic acid. 1st communication: absorption, distribution, metabolism and excretion in mice, rats, dogs and monkeys after single administration of 14C-labeled compound.Arzneimittelforschung. 1990 Jul;40(7):800-5. Arzneimittelforschung. 1990. PMID: 2222556
-
Intra-Articular Injection of BPC 157 for Multiple Types of Knee Pain.Altern Ther Health Med. 2021 Jul;27(4):8-13. Altern Ther Health Med. 2021. PMID: 34324435
-
Pharmacokinetics and metabolism of the new thromboxane A2 receptor antagonist ramatroban in animals. 1st communication: absorption, concentrations in plasma, metabolism, and excretion after single administration to rats and dogs.Arzneimittelforschung. 1997 Aug;47(8):928-38. Arzneimittelforschung. 1997. PMID: 9296279
Cited by
-
Stable Gastric Pentadecapeptide BPC 157 as a Therapy and Safety Key: A Special Beneficial Pleiotropic Effect Controlling and Modulating Angiogenesis and the NO-System.Pharmaceuticals (Basel). 2025 Jun 19;18(6):928. doi: 10.3390/ph18060928. Pharmaceuticals (Basel). 2025. PMID: 40573323 Free PMC article. Review.
-
Regeneration or Risk? A Narrative Review of BPC-157 for Musculoskeletal Healing.Curr Rev Musculoskelet Med. 2025 Aug 12. doi: 10.1007/s12178-025-09990-7. Online ahead of print. Curr Rev Musculoskelet Med. 2025. PMID: 40789979 Review. No abstract available.
-
Multifunctionality and Possible Medical Application of the BPC 157 Peptide-Literature and Patent Review.Pharmaceuticals (Basel). 2025 Jan 30;18(2):185. doi: 10.3390/ph18020185. Pharmaceuticals (Basel). 2025. PMID: 40005999 Free PMC article. Review.
-
Emerging Use of BPC-157 in Orthopaedic Sports Medicine: A Systematic Review.HSS J. 2025 Jul 31:15563316251355551. doi: 10.1177/15563316251355551. Online ahead of print. HSS J. 2025. PMID: 40756949 Free PMC article. Review.
-
Protective Effects of BPC 157 on Liver, Kidney, and Lung Distant Organ Damage in Rats with Experimental Lower-Extremity Ischemia-Reperfusion Injury.Medicina (Kaunas). 2025 Feb 8;61(2):291. doi: 10.3390/medicina61020291. Medicina (Kaunas). 2025. PMID: 40005408 Free PMC article.
References
LinkOut - more resources
Full Text Sources

