The significance of glycolysis in tumor progression and its relationship with the tumor microenvironment
- PMID: 36588722
- PMCID: PMC9795015
- DOI: 10.3389/fphar.2022.1091779
The significance of glycolysis in tumor progression and its relationship with the tumor microenvironment
Abstract
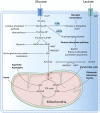
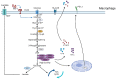
It is well known that tumor cells rely mainly on aerobic glycolysis for energy production even in the presence of oxygen, and glycolysis is a known modulator of tumorigenesis and tumor development. The tumor microenvironment (TME) is composed of tumor cells, various immune cells, cytokines, and extracellular matrix, among other factors, and is a complex niche supporting the survival and development of tumor cells and through which they interact and co-evolve with other tumor cells. In recent years, there has been a renewed interest in glycolysis and the TME. Many studies have found that glycolysis promotes tumor growth, metastasis, and chemoresistance, as well as inhibiting the apoptosis of tumor cells. In addition, lactic acid, a metabolite of glycolysis, can also accumulate in the TME, leading to reduced extracellular pH and immunosuppression, and affecting the TME. This review discusses the significance of glycolysis in tumor development, its association with the TME, and potential glycolysis-targeted therapies, to provide new ideas for the clinical treatment of tumors.
Keywords: glycolysis; immune cells; inflammatory factors; targeted therap; tumor microenvironment.
Copyright © 2022 Zhou, Duan, Li, Ge, Wei and Kong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Tumor Microenvironment Lactate: Is It a Cancer Progression Marker, Immunosuppressant, and Therapeutic Target?Molecules. 2025 Apr 15;30(8):1763. doi: 10.3390/molecules30081763. Molecules. 2025. PMID: 40333742 Free PMC article. Review.
-
Lactic acid: a narrative review of a promoter of the liver cancer microenvironment.J Gastrointest Oncol. 2024 Jun 30;15(3):1282-1296. doi: 10.21037/jgo-24-368. Epub 2024 Jun 13. J Gastrointest Oncol. 2024. PMID: 38989406 Free PMC article. Review.
-
Metabolic Interplay in the Tumor Microenvironment: Implications for Immune Function and Anticancer Response.Curr Issues Mol Biol. 2023 Dec 5;45(12):9753-9767. doi: 10.3390/cimb45120609. Curr Issues Mol Biol. 2023. PMID: 38132455 Free PMC article. Review.
-
Tumor Microenvironment: Lactic Acid Promotes Tumor Development.J Immunol Res. 2022 Jun 12;2022:3119375. doi: 10.1155/2022/3119375. eCollection 2022. J Immunol Res. 2022. PMID: 35733921 Free PMC article. Review.
-
Glycolysis in the tumor microenvironment: a driver of cancer progression and a promising therapeutic target.Front Cell Dev Biol. 2024 Jun 12;12:1416472. doi: 10.3389/fcell.2024.1416472. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38933335 Free PMC article. Review.
Cited by
-
Mitochondrial Plasticity and Glucose Metabolic Alterations in Human Cancer under Oxidative Stress-From Viewpoints of Chronic Inflammation and Neutrophil Extracellular Traps (NETs).Int J Mol Sci. 2024 Aug 30;25(17):9458. doi: 10.3390/ijms25179458. Int J Mol Sci. 2024. PMID: 39273403 Free PMC article. Review.
-
Proteomics Profiling of Bladder Cancer Tissues from Early to Advanced Stages Reveals NNMT and GALK1 as Biomarkers for Early Detection and Prognosis of BCa.Int J Mol Sci. 2023 Oct 6;24(19):14938. doi: 10.3390/ijms241914938. Int J Mol Sci. 2023. PMID: 37834386 Free PMC article.
-
DeepGR: a deep-learning prognostic model based on glycolytic radiomics for non-small cell lung cancer.Transl Lung Cancer Res. 2024 Oct 31;13(10):2746-2760. doi: 10.21037/tlcr-24-716. Epub 2024 Oct 17. Transl Lung Cancer Res. 2024. PMID: 39507025 Free PMC article.
-
Tumor Microenvironment Responsive Key Nanomicelles for Effective Against Invasion and Metastasis in Ovarian Cancer Using Mice Model.Int J Nanomedicine. 2025 Jan 7;20:215-238. doi: 10.2147/IJN.S470219. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39802386 Free PMC article.
-
Competing endogenous RNAs (ceRNAs) and drug resistance to cancer therapy.Cancer Drug Resist. 2024 Sep 25;7:37. doi: 10.20517/cdr.2024.66. eCollection 2024. Cancer Drug Resist. 2024. PMID: 39403602 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

