C188-9, a specific inhibitor of STAT3 signaling, prevents thermal burn-induced skeletal muscle wasting in mice
- PMID: 36588738
- PMCID: PMC9800842
- DOI: 10.3389/fphar.2022.1031906
C188-9, a specific inhibitor of STAT3 signaling, prevents thermal burn-induced skeletal muscle wasting in mice
Abstract
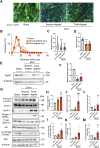
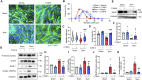
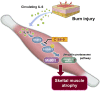
Burn injury is the leading cause of death and disability worldwide and places a tremendous economic burden on society. Systemic inflammatory responses induced by thermal burn injury can cause muscle wasting, a severe involuntary loss of skeletal muscle that adversely affects the survival and functional outcomes of these patients. Currently, no pharmacological interventions are available for the treatment of thermal burn-induced skeletal muscle wasting. Elevated levels of inflammatory cytokines, such as interleukin-6 (IL-6), are important hallmarks of severe burn injury. The levels of signal transducer and activator of transcription 3 (STAT3)-a downstream component of IL-6 inflammatory signaling-are elevated with muscle wasting in various pro-catabolic conditions, and STAT3 has been implicated in the regulation of skeletal muscle atrophy. Here, we tested the effects of the STAT3-specific signaling inhibitor C188-9 on thermal burn injury-induced skeletal muscle wasting in vivo and on C2C12 myotube atrophy in vitro after the administration of plasma from burn model mice. In mice, thermal burn injury severity dependently increased IL-6 in the plasma and tibialis anterior muscles and activated the STAT3 (increased ratio of phospho-STAT3/STAT3) and ubiquitin-proteasome proteolytic pathways (increased Atrogin-1/MAFbx and MuRF1). These effects resulted in skeletal muscle atrophy and reduced grip strength. In murine C2C12 myotubes, plasma from burn mice activated the same inflammatory and proteolytic pathways, leading to myotube atrophy. In mice with burn injury, the intraperitoneal injection of C188-9 (50 mg/kg) reduced activation of the STAT3 and ubiquitin-proteasome proteolytic pathways, reversed skeletal muscle atrophy, and increased grip strength. Similarly, pretreatment of murine C2C12 myotubes with C188-9 (10 µM) reduced activation of the same inflammatory and proteolytic pathways, and ameliorated myotube atrophy induced by plasma taken from burn model mice. Collectively, these results indicate that pharmacological inhibition of STAT3 signaling may be a novel therapeutic strategy for thermal burn-induced skeletal muscle wasting.
Keywords: humoral factor; hyper catabolism; interleukin-6; pharmacological intervention; skeletal muscle atrophy; systemic inflammatory response syndrome; ubiquitin proteasome pathway.
Copyright © 2022 Ono, Saito, Sakamoto, Maejima, Misaka, Shimomura, Nakanishi, Inoue and Kotani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
TAK-242, a specific inhibitor of Toll-like receptor 4 signalling, prevents endotoxemia-induced skeletal muscle wasting in mice.Sci Rep. 2020 Jan 20;10(1):694. doi: 10.1038/s41598-020-57714-3. Sci Rep. 2020. PMID: 31959927 Free PMC article.
-
Cryptotanshinone prevents muscle wasting in CT26-induced cancer cachexia through inhibiting STAT3 signaling pathway.J Ethnopharmacol. 2020 Oct 5;260:113066. doi: 10.1016/j.jep.2020.113066. Epub 2020 Jun 4. J Ethnopharmacol. 2020. PMID: 32505837
-
Sepsis induces interleukin 6, gp130/JAK2/STAT3, and muscle wasting.J Cachexia Sarcopenia Muscle. 2022 Feb;13(1):713-727. doi: 10.1002/jcsm.12867. Epub 2021 Nov 24. J Cachexia Sarcopenia Muscle. 2022. PMID: 34821076 Free PMC article.
-
PI3 kinase regulation of skeletal muscle hypertrophy and atrophy.Curr Top Microbiol Immunol. 2010;346:267-78. doi: 10.1007/82_2010_78. Curr Top Microbiol Immunol. 2010. PMID: 20593312 Review.
-
Molecular mechanisms of post-burn muscle wasting and the therapeutic potential of physical exercise.J Cachexia Sarcopenia Muscle. 2023 Apr;14(2):758-770. doi: 10.1002/jcsm.13188. Epub 2023 Feb 9. J Cachexia Sarcopenia Muscle. 2023. PMID: 36760077 Free PMC article. Review.
Cited by
-
Inflammation-related proteomics demonstrate landscape of fracture blister fluid in patients with acute compartment syndrome.Front Immunol. 2023 Apr 6;14:1161479. doi: 10.3389/fimmu.2023.1161479. eCollection 2023. Front Immunol. 2023. PMID: 37090725 Free PMC article.
-
A scoping review of preclinical intensive care unit-acquired weakness models.Front Physiol. 2024 Oct 2;15:1423567. doi: 10.3389/fphys.2024.1423567. eCollection 2024. Front Physiol. 2024. PMID: 39416383 Free PMC article.
-
JAK2/STAT3 Signaling Pathway Modulates Acute Methylmercury Toxicity in the Mouse Astrocyte C8-D1A Cell Line.Neurochem Res. 2025 Aug 13;50(4):265. doi: 10.1007/s11064-025-04507-7. Neurochem Res. 2025. PMID: 40802001 Free PMC article.
-
Protective effects of Lactobacillus plantarum strain against protein malnutrition-induced muscle atrophy and bone loss in juvenile mice.PLoS One. 2025 Jan 16;20(1):e0317197. doi: 10.1371/journal.pone.0317197. eCollection 2025. PLoS One. 2025. PMID: 39820793 Free PMC article.
-
Effects of Thyroid Hormones on Cellular Development in Human Ovarian Granulosa Tumor Cells (KGN).Reprod Sci. 2025 May;32(5):1545-1556. doi: 10.1007/s43032-024-01721-6. Epub 2024 Oct 15. Reprod Sci. 2025. PMID: 39407059
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

