Myelin lipid metabolism and its role in myelination and myelin maintenance
- PMID: 36588745
- PMCID: PMC9800635
- DOI: 10.1016/j.xinn.2022.100360
Myelin lipid metabolism and its role in myelination and myelin maintenance
Abstract
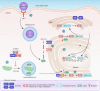
Myelin is a specialized cell membrane indispensable for rapid nerve conduction. The high abundance of membrane lipids is one of myelin's salient features that contribute to its unique role as an insulator that electrically isolates nerve fibers across their myelinated surface. The most abundant lipids in myelin include cholesterol, glycosphingolipids, and plasmalogens, each playing critical roles in myelin development as well as function. This review serves to summarize the role of lipid metabolism in myelination and myelin maintenance, as well as the molecular determinants of myelin lipid homeostasis, with an emphasis on findings from genetic models. In addition, the implications of myelin lipid dysmetabolism in human diseases are highlighted in the context of hereditary leukodystrophies and neuropathies as well as acquired disorders such as Alzheimer's disease.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Tasaki I. The electro-saltatory transmission of the nerve impulse and the effect of narcosis upon the nerve fiber. American Journal of Physiology-Legacy Content. 1939;127:211–227.
-
- Huxley A.F., Stämpfli R. Evidence for saltatory conduction in peripheral myelinated nerve fibres. J. Physiol. 1949;108:315–339. - PubMed
-
- Yeung M.S.Y., Zdunek S., Frisén J. Dynamics of oligodendrocyte generation and myelination in the human brain. Cell. 2014;159:766–774. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

