Progressive multifocal leukoencephalopathy genetic risk variants for pharmacovigilance of immunosuppressant therapies
- PMID: 36588876
- PMCID: PMC9795231
- DOI: 10.3389/fneur.2022.1016377
Progressive multifocal leukoencephalopathy genetic risk variants for pharmacovigilance of immunosuppressant therapies
Abstract
Background: Progressive multifocal leukoencephalopathy (PML) is a rare and often lethal brain disorder caused by the common, typically benign polyomavirus 2, also known as JC virus (JCV). In a small percentage of immunosuppressed individuals, JCV is reactivated and infects the brain, causing devastating neurological defects. A wide range of immunosuppressed groups can develop PML, such as patients with: HIV/AIDS, hematological malignancies (e.g., leukemias, lymphomas, and multiple myeloma), autoimmune disorders (e.g., psoriasis, rheumatoid arthritis, and systemic lupus erythematosus), and organ transplants. In some patients, iatrogenic (i.e., drug-induced) PML occurs as a serious adverse event from exposure to immunosuppressant therapies used to treat their disease (e.g., hematological malignancies and multiple sclerosis). While JCV infection and immunosuppression are necessary, they are not sufficient to cause PML.
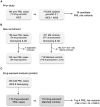
Methods: We hypothesized that patients may also have a genetic susceptibility from the presence of rare deleterious genetic variants in immune-relevant genes (e.g., those that cause inborn errors of immunity). In our prior genetic study of 184 PML cases, we discovered 19 candidate PML risk variants. In the current study of another 152 cases, we validated 4 of 19 variants in both population controls (gnomAD 3.1) and matched controls (JCV+ multiple sclerosis patients on a PML-linked drug ≥ 2 years).
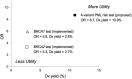
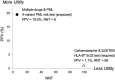
Results: The four variants, found in immune system genes with strong biological links, are: C8B, 1-57409459-C-A, rs139498867; LY9 (alias SLAMF3), 1-160769595-AG-A, rs763811636; FCN2, 9-137779251-G-A, rs76267164; STXBP2, 19-7712287-G-C, rs35490401. Carriers of any one of these variants are shown to be at high risk of PML when drug-exposed PML cases are compared to drug-exposed matched controls: P value = 3.50E-06, OR = 8.7 [3.7-20.6]. Measures of clinical validity and utility compare favorably to other genetic risk tests, such as BRCA1 and BRCA2 screening for breast cancer risk and HLA-B*15:02 pharmacogenetic screening for pharmacovigilance of carbamazepine to prevent Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis.
Conclusion: For the first time, a PML genetic risk test can be implemented for screening patients taking or considering treatment with a PML-linked drug in order to decrease the incidence of PML and enable safer use of highly effective therapies used to treat their underlying disease.
Keywords: JC virus; PML; immunodeficiency; multiple sclerosis; natalizumab; pharmacovigilance; progressive multifocal leukoencephalopathy; serious adverse event.
Copyright © 2022 Hatchwell, Smith, Jalilzadeh, Bruno, Taoufik, Hendel-Chavez, Liblau, Brassat, Martin-Blondel, Wiendl, Schwab, Cortese, Monaco, Imberti, Capra, Oksenberg, Gasnault, Stankoff, Richmond, Rancour, Koralnik, Hanson, Major, Chow and Eis.
Conflict of interest statement
Authors CB and CC are employed by Emerald Lake Safety LLC. Authors EH, ES, PE, and SJ are employed by Population Bio, Inc. Author TR is employed by Richmond Bioinformatics Consulting and author DR is employed by Lytic Solutions, LLC. Authors HW, IK, NS, and RL received funding from PML Screening, LLC to partially offset the costs for collection, and clinical characterization of patient samples used in the research. Authors EH, ES, PE, and YT are inventors of genetic screening methods for PML risk and have issued and pending patents related to this work. Applicants/Assignees on issued patents are: PML Screening, LLC, Newport Beach, CA (US), a joint venture between Population Bio, Inc. and Emerald Lake Safety LLC; Université Paris-Saclay, Gif sur Yvette (FR); The Assistance Publique-Hôpitaux de Paris (APHP), Paris (FR); and The Institut National de la Santé et de la Recherche Médicale (INSERM), Paris (FR). Author IC is a shareholder in Keires AG, Nouscom AG, and PDC*line Pharma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from PML Screening, LLC. The funders had the following involvement with the study: conception and design of the study, laboratory experiments, data analysis and interpretation, and wrote the manuscript.
Figures
Comment in
-
Commentary: Progressive multifocal leukoencephalopathy genetic risk variants for pharmacovigilance of immunosuppressant therapies.Front Neurol. 2023 Mar 16;14:1146027. doi: 10.3389/fneur.2023.1146027. eCollection 2023. Front Neurol. 2023. PMID: 37006492 Free PMC article. No abstract available.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

