Limitations of current chemotherapy and future of nanoformulation-based AmB delivery for visceral leishmaniasis-An updated review
- PMID: 36588956
- PMCID: PMC9794769
- DOI: 10.3389/fbioe.2022.1016925
Limitations of current chemotherapy and future of nanoformulation-based AmB delivery for visceral leishmaniasis-An updated review
Abstract
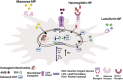
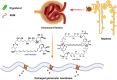
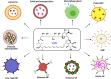
Visceral leishmaniasis (VL) is the most lethal of all leishmaniasis diseasesand the second most common parasiticdisease after malaria and,still, categorized as a neglected tropical disease (NTD). According to the latest WHO study, >20 Leishmania species spread 0.7-1.0 million new cases of leishmaniasis each year. VL is caused by the genus, Leishmania donovani (LD), which affects between 50,000 and 90,000 people worldwide each year. Lack of new drug development, increasing drug resistance, toxicity and high cost even with the first line of treatmentof Amphotericin B (AmB), demands new formulation for treatment of VLFurther the lack of a vaccine, allowedthe researchers to develop nanofomulation-based AmB for improved delivery. The limitation of AmB is its kidney and liver toxicity which forced the development of costly liposomal AmB (AmBisome) nanoformulation. Success of AmBisome have inspired and attracted a wide range of AmB nanoformulations ranging from polymeric, solid lipid, liposomal/micellar, metallic, macrophage receptor-targetednanoparticles (NP) and even with sophisticated carbon/quantum dot-based AmBnano delivery systems. Notably, NP-based AmB delivery has shown increased efficacy due to increased uptake, on-target delivery and synergistic impact of NP and AmB. In this review, we have discussed the different forms of leishmaniasis disease and their current treatment options with limitations. The discovery, mechanism of action of AmB, clinical status of AmB and improvement with AmBisome over fungizone (AmB-deoxycholate)for VL treatment was further discussed. At last, the development of various AmB nanoformulation was discussed along with its adavantages over traditional chemotherapy-based delivery.
Keywords: amphotericin B; clinical status; drug delivery; leishmaniasis; nanoparticle.
Copyright © 2022 Kumar, Kumar, Singh, Khajuria, Patel, Rajana, Mandal and Velayutham.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ahmad A., Wei Y., Syed F., Khan S., Khan G. M., Tahir K., et al. (2016). Isatis tinctoria mediated synthesis of amphotericin B-bound silver nanoparticles with enhanced photoinduced antileishmanial activity: A novel green approach. J. Photochem. Photobiol. B Biol. 161, 17–24. 10.1016/j.jphotobiol.2016.05.003 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

