Rhizospheric microbiomics integrated with plant transcriptomics provides insight into the Cd response mechanisms of the newly identified Cd accumulator Dahlia pinnata
- PMID: 36589044
- PMCID: PMC9798219
- DOI: 10.3389/fpls.2022.1091056
Rhizospheric microbiomics integrated with plant transcriptomics provides insight into the Cd response mechanisms of the newly identified Cd accumulator Dahlia pinnata
Abstract
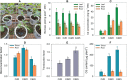
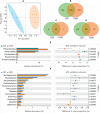
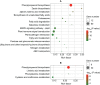
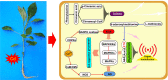
Phytoremediation that depends on excellent plant resources and effective enhancing measures is important for remediating heavy metal-contaminated soils. This study investigated the cadmium (Cd) tolerance and accumulation characteristics of Dahlia pinnata Cav. to evaluate its Cd phytoremediation potential. Testing in soils spiked with 5-45 mg kg-1 Cd showed that D. pinnata has a strong Cd tolerance capacity and appreciable shoot Cd bioconcentration factors (0.80-1.32) and translocation factors (0.81-1.59), indicating that D. pinnata can be defined as a Cd accumulator. In the rhizosphere, Cd stress (45 mg kg-1 Cd) did not change the soil physicochemical properties but influenced the bacterial community composition compared to control conditions. Notably, the increased abundance of the bacterial phylum Patescibacteria and the dominance of several Cd-tolerant plant growth-promoting rhizobacteria (e.g., Sphingomonas, Gemmatimonas, Bryobacter, Flavisolibacter, Nocardioides, and Bradyrhizobium) likely facilitated Cd tolerance and accumulation in D. pinnata. Comparative transcriptomic analysis showed that Cd significantly induced (P < 0.001) the expression of genes involved in lignin synthesis in D. pinnata roots and leaves, which are likely to fix Cd2+ to the cell wall and inhibit Cd entry into the cytoplasm. Moreover, Cd induced a sophisticated signal transduction network that initiated detoxification processes in roots as well as ethylene synthesis from methionine metabolism to regulate Cd responses in leaves. This study suggests that D. pinnata can be potentially used for phytoextraction and improves our understanding of Cd-response mechanisms in plants from rhizospheric and molecular perspectives.
Keywords: Dahlia pinnata; heavy metal contamination; phytoextraction; rhizobacteria; signal transduction.
Copyright © 2022 Li, Li, Jin, Chen, Zhao, Qin, Yang and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Physiological and rhizospheric response characteristics to cadmium of a newly identified cadmium accumulator Coreopsis grandiflora Hogg. (Asteraceae).Ecotoxicol Environ Saf. 2022 Aug;241:113739. doi: 10.1016/j.ecoenv.2022.113739. Epub 2022 Jun 14. Ecotoxicol Environ Saf. 2022. PMID: 35714481
-
Phytoremediation potential evaluation of three rhubarb species and comparative analysis of their rhizosphere characteristics in a Cd- and Pb-contaminated soil.Chemosphere. 2022 Jun;296:134045. doi: 10.1016/j.chemosphere.2022.134045. Epub 2022 Feb 17. Chemosphere. 2022. PMID: 35183585
-
Polyaspartic acid enhances the Cd phytoextraction efficiency of Bidens pilosa by remolding the rhizospheric environment and reprogramming plant metabolism.Chemosphere. 2022 Nov;307(Pt 3):136068. doi: 10.1016/j.chemosphere.2022.136068. Epub 2022 Aug 16. Chemosphere. 2022. PMID: 35985384
-
Implications of metal accumulation mechanisms to phytoremediation.Environ Sci Pollut Res Int. 2009 Mar;16(2):162-75. doi: 10.1007/s11356-008-0079-z. Epub 2008 Dec 6. Environ Sci Pollut Res Int. 2009. PMID: 19067014 Review.
-
Advances in the application of plant growth-promoting rhizobacteria in phytoremediation of heavy metals.Rev Environ Contam Toxicol. 2013;223:33-52. doi: 10.1007/978-1-4614-5577-6_2. Rev Environ Contam Toxicol. 2013. PMID: 23149811 Review.
Cited by
-
Exogenous nano-silicon enhances the ability of intercropped faba bean to alleviate cadmium toxicity and resist Fusarium wilt.J Nanobiotechnology. 2025 Apr 1;23(1):262. doi: 10.1186/s12951-025-03330-0. J Nanobiotechnology. 2025. PMID: 40170068 Free PMC article.
-
Community dynamics in rhizosphere bacteria affected the adaptive growth of wheat in cadmium-contaminated soils.Physiol Mol Biol Plants. 2024 Nov;30(11):1841-1852. doi: 10.1007/s12298-024-01532-8. Epub 2024 Nov 28. Physiol Mol Biol Plants. 2024. PMID: 39687698
-
Three local plants adapt to ecological restoration of abandoned lead-zinc mines through assembly of rhizosphere bacterial communities.Front Microbiol. 2025 Feb 10;16:1533965. doi: 10.3389/fmicb.2025.1533965. eCollection 2025. Front Microbiol. 2025. PMID: 39996083 Free PMC article.
-
A comparative analysis of the rhizosphere microbial communities among three species of the Salix genus.PeerJ. 2025 Mar 28;13:e19182. doi: 10.7717/peerj.19182. eCollection 2025. PeerJ. 2025. PMID: 40166043 Free PMC article.
-
Biological Decline of Alfalfa Is Accompanied by Negative Succession of Rhizosphere Soil Microbial Communities.Plants (Basel). 2024 Sep 16;13(18):2589. doi: 10.3390/plants13182589. Plants (Basel). 2024. PMID: 39339564 Free PMC article.
References
-
- Baker A. J. M. (1981). Accumulators and excluders -strategies in the response of plants to heavy metals. J. Plant Nutr. 3, 643–654. doi: 10.1080/01904168109362867 - DOI
LinkOut - more resources
Full Text Sources

