Aboveground herbivory does not affect mycorrhiza-dependent nitrogen acquisition from soil but inhibits mycorrhizal network-mediated nitrogen interplant transfer in maize
- PMID: 36589048
- PMCID: PMC9795027
- DOI: 10.3389/fpls.2022.1080416
Aboveground herbivory does not affect mycorrhiza-dependent nitrogen acquisition from soil but inhibits mycorrhizal network-mediated nitrogen interplant transfer in maize
Abstract
Arbuscular mycorrhizal fungi (AMF) are considered biofertilizers for sustainable agriculture due to their ability to facilitate plant uptake of important mineral elements, such as nitrogen (N). However, plant mycorrhiza-dependent N uptake and interplant transfer may be highly context-dependent, and whether it is affected by aboveground herbivory remains largely unknown. Here, we used 15N labeling and tracking to examine the effect of aboveground insect herbivory by Spodoptera frugiperda on mycorrhiza-dependent N uptake in maize (Zea mays L.). To minimize consumption differences and 15N loss due to insect chewing, insect herbivory was simulated by mechanical wounding and oral secretion of S. frugiperda larvae. Inoculation with Rhizophagus irregularis (Rir) significantly improved maize growth, and N/P uptake. The 15N labeling experiment showed that maize plants absorbed N from soils via the extraradical mycelium of mycorrhizal fungi and from neighboring plants transferred by common mycorrhizal networks (CMNs). Simulated aboveground leaf herbivory did not affect mycorrhiza-mediated N acquisition from soil. However, CMN-mediated N transfer from neighboring plants was blocked by leaf simulated herbivory. Our findings suggest that aboveground herbivory inhibits CMN-mediated N transfer between plants but does not affect N acquisition from soil solutions via extraradical mycorrhizal mycelium.
Keywords: Zea mays; common mycorrhizal networks; insect herbivory; mycorrhiza; nitrogen.
Copyright © 2022 He, Lin, Zhang, Tong, Ding, Yao, Liu, Zeng, Chen and Song.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
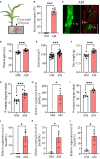




 , N transferred from a CMN-connected neighboring plant was blocked.
, N transferred from a CMN-connected neighboring plant was blocked.Similar articles
-
Increased maize growth and P uptake promoted by arbuscular mycorrhizal fungi coincide with higher foliar herbivory and larval biomass of the Fall Armyworm Spodoptera frugiperda.Mycorrhiza. 2019 Nov;29(6):615-622. doi: 10.1007/s00572-019-00920-3. Epub 2019 Nov 14. Mycorrhiza. 2019. PMID: 31724088
-
Maize mycorrhizas decrease the susceptibility of the foliar insect herbivore Spodoptera frugiperda to its homologous nucleopolyhedrovirus.Pest Manag Sci. 2021 Oct;77(10):4701-4708. doi: 10.1002/ps.6511. Epub 2021 Jun 22. Pest Manag Sci. 2021. PMID: 34129282
-
Cadmium transfer between maize and soybean plants via common mycorrhizal networks.Ecotoxicol Environ Saf. 2022 Mar 1;232:113273. doi: 10.1016/j.ecoenv.2022.113273. Epub 2022 Feb 3. Ecotoxicol Environ Saf. 2022. PMID: 35123184
-
Interplant signalling through hyphal networks.New Phytol. 2015 Mar;205(4):1448-1453. doi: 10.1111/nph.13115. Epub 2014 Nov 24. New Phytol. 2015. PMID: 25421970 Review.
-
Terpenoids in plant and arbuscular mycorrhiza-reinforced defence against herbivorous insects.Ann Bot. 2017 Mar 1;119(5):791-801. doi: 10.1093/aob/mcw263. Ann Bot. 2017. PMID: 28087662 Free PMC article. Review.
Cited by
-
Mycorrhizae in mine wasteland reclamation.Heliyon. 2024 Jun 17;10(13):e33141. doi: 10.1016/j.heliyon.2024.e33141. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39035525 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

