Roles of plastid-located phosphate transporters in carotenoid accumulation
- PMID: 36589064
- PMCID: PMC9798012
- DOI: 10.3389/fpls.2022.1059536
Roles of plastid-located phosphate transporters in carotenoid accumulation
Abstract
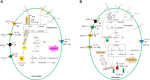
Enhanced carotenoid accumulation in plants is crucial for the nutritional and health demands of the human body since these beneficial substances are acquired through dietary intake. Plastids are the major organelles to accumulate carotenoids in plants and it is reported that manipulation of a single plastid phosphate transporter gene enhances carotenoid accumulation. Amongst all phosphate transport proteins including phosphate transporters (PHTs), plastidial phosphate translocators (pPTs), PHOSPHATE1 (PHO1), vacuolar phosphate efflux transporter (VPE), and Sulfate transporter [SULTR]-like phosphorus distribution transporter (SPDT) in plants, plastidic PHTs (PHT2 & PHT4) are found as the only clade that is plastid located, and manipulation of which affects carotenoid accumulation. Manipulation of a single chromoplast PHT (PHT4;2) enhances carotenoid accumulation, whereas manipulation of a single chloroplast PHT has no impact on carotenoid accumulation. The underlying mechanism is mainly attributed to their different effects on plastid orthophosphate (Pi) concentration. PHT4;2 is the only chromoplast Pi efflux transporter, and manipulating this single chromoplast PHT significantly regulates chromoplast Pi concentration. This variation subsequently modulates the carotenoid accumulation by affecting the supply of glyceraldehyde 3-phosphate, a substrate for carotenoid biosynthesis, by modulating the transcript abundances of carotenoid biosynthesis limited enzyme genes, and by regulating chromoplast biogenesis (facilitating carotenoid storage). However, at least five orthophosphate influx PHTs are identified in the chloroplast, and manipulating one of the five does not substantially modulate the chloroplast Pi concentration in a long term due to their functional redundancy. This stable chloroplast Pi concentration upon one chloroplast PHT absence, therefore, is unable to modulate Pi-involved carotenoid accumulation processes and finally does affect carotenoid accumulation in photosynthetic tissues. Despite these advances, several cases including the precise location of plastid PHTs, the phosphate transport direction mediated by these plastid PHTs, the plastid PHTs participating in carotenoid accumulation signal pathway, the potential roles of these plastid PHTs in leaf carotenoid accumulation, and the roles of these plastid PHTs in other secondary metabolites are waiting for further research. The clarification of the above-mentioned cases is beneficial for breeding high-carotenoid accumulation plants (either in photosynthetic or non-photosynthetic edible parts of plants) through the gene engineering of these transporters.
Keywords: carotenoid accumulation; chloroplast; chromoplast; phosphate transporter; plastid.
Copyright © 2022 Hao, Zhou, Huang, Wang, Li, Guo and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

