Tissue specific signature of HHV-6 infection in ME/CFS
- PMID: 36589231
- PMCID: PMC9795011
- DOI: 10.3389/fmolb.2022.1044964
Tissue specific signature of HHV-6 infection in ME/CFS
Abstract
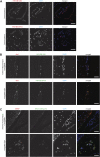
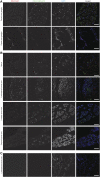
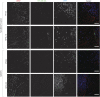
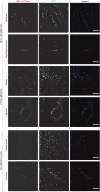
First exposure to various human herpesviruses (HHVs) including HHV-6, HCMV and EBV does not cause a life-threatening disease. In fact, most individuals are frequently unaware of their first exposure to such pathogens. These herpesviruses acquire lifelong latency in the human body where they show minimal genomic activity required for their survival. We hypothesized that it is not the latency itself but a timely, regionally restricted viral reactivation in a sub-set of host cells that plays a key role in disease development. HHV-6 (HHV-6A and HHV-6B) and HHV-7 are unique HHVs that acquire latency by integration of the viral genome into sub-telomeric region of human chromosomes. HHV-6 reactivation has been linked to Alzheimer's Disease, Chronic Fatigue Syndrome, and many other diseases. However, lack of viral activity in commonly tested biological materials including blood or serum strongly suggests tissue specific localization of active HHV-6 genome. Here in this paper, we attempted to analyze active HHV-6 transcripts in postmortem tissue biopsies from a small cohort of ME/CFS patients and matched controls by fluorescence in situ hybridization using a probe against HHV-6 microRNA (miRNA), miR-aU14. Our results show abundant viral miRNA in various regions of the human brain and associated neuronal tissues including the spinal cord that is only detected in ME/CFS patients and not in controls. Our findings provide evidence of tissue-specific active HHV-6 and EBV infection in ME/CFS, which along with recent work demonstrating a possible relationship between herpesvirus infection and ME/CFS, provide grounds for renewed discussion on the role of herpesviruses in ME/CFS.
Keywords: EBV; HHV-6; ME/CFS; epstein-barr virus; herpesvirus; viral pathology.
Copyright © 2022 Kasimir, Toomey, Liu, Kaiping, Ariza and Prusty.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources

