Mathematical modeling of antihypertensive therapy
- PMID: 36589434
- PMCID: PMC9795234
- DOI: 10.3389/fphys.2022.1070115
Mathematical modeling of antihypertensive therapy
Abstract
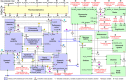
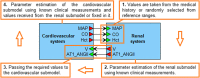
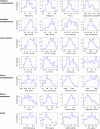
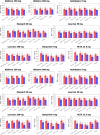
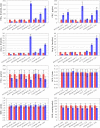
Hypertension is a multifactorial disease arising from complex pathophysiological pathways. Individual characteristics of patients result in different responses to various classes of antihypertensive medications. Therefore, evaluating the efficacy of therapy based on in silico predictions is an important task. This study is a continuation of research on the modular agent-based model of the cardiovascular and renal systems (presented in the previously published article). In the current work, we included in the model equations simulating the response to antihypertensive therapies with different mechanisms of action. For this, we used the pharmacodynamic effects of the angiotensin II receptor blocker losartan, the calcium channel blocker amlodipine, the angiotensin-converting enzyme inhibitor enalapril, the direct renin inhibitor aliskiren, the thiazide diuretic hydrochlorothiazide, and the β-blocker bisoprolol. We fitted therapy parameters based on known clinical trials for all considered medications, and then tested the model's ability to show reasonable dynamics (expected by clinical observations) after treatment with individual drugs and their dual combinations in a group of virtual patients with hypertension. The extended model paves the way for the next step in personalized medicine that is adapting the model parameters to a real patient and predicting his response to antihypertensive therapy. The model is implemented in the BioUML software and is available at https://gitlab.sirius-web.org/virtual-patient/antihypertensive-treatment-modeling.
Keywords: agent-based modular model; antihypertensive therapy; blood pressure regulation; cardiovascular system; mathematical modeling; renal system.
Copyright © 2022 Kutumova, Kiselev, Sharipov, Lifshits and Kolpakov.
Conflict of interest statement
Authors EK, IK, RS, and FK were employed by Biosoft.Ru, Ltd. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Abate G., Zito M., Carbonin P. U., Cocchi A., Cucinotta D., Manopulo R., et al. (1998). Pinacidil and hydrochlorothiazide alone or in combination in the treatment of hypertension in the elderly. Curr. Ther. Res. 59 (1), 62–71. 10.1016/s0011-393x(98)85025-x - DOI
-
- Alwi I. (2010). Diagnosis and management of cardiogenic pulmonary edema. Acta Med. Indones. 42 (3), 176–184. - PubMed
LinkOut - more resources
Full Text Sources

