Inhibition of PFKFB Preserves Intestinal Barrier Function in Sepsis by Inhibiting NLRP3/GSDMD
- PMID: 36589684
- PMCID: PMC9803577
- DOI: 10.1155/2022/8704016
Inhibition of PFKFB Preserves Intestinal Barrier Function in Sepsis by Inhibiting NLRP3/GSDMD
Abstract
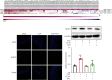
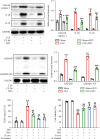
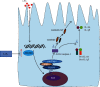
Intestinal barrier dysfunction is associated with the occurrence and development of sepsis. Further, aerobic glycolysis plays an essential role in inflammation and cell death. This study is aimed at investigating the protective effect and mechanism of PFKFB3 inhibition on intestinal barrier dysfunction in sepsis mice. Sepsis mouse models were established by cecal ligation and puncture (CLP) in wild-type mice and Gsdmd-/- mice. The results showed that the expression of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) in the small intestines was significantly upregulated in sepsis. 3-(3-Pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO), the specific inhibitor of PFKFB3, and Gsdmd gene knockout significantly inhibited the inflammatory response and cell death caused by sepsis, thus alleviating intestinal damage and barrier dysfunction. 3PO was also shown to significantly inhibit oxidative stress and NLRP3/caspase-1/GSDMD-dependent cell pyroptosis in the small intestines. The in vitro studies revealed that 3PO reduced NLRP3/caspase-1/GSDMD-dependent cell pyroptosis by inhibiting ROS. Taken together, our results suggest that PFKFB3 is involved in inflammation, oxidative stress, and pyroptosis during sepsis and enhances intestinal damage, which may provide important clues about the potential targets to be exploited in this highly lethal disease.
Copyright © 2022 Yongsheng Zhang et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

