Sulfatase-2 from Cancer Associated Fibroblasts: An Environmental Target for Hepatocellular Carcinoma?
- PMID: 36589727
- PMCID: PMC9801184
- DOI: 10.1159/000525375
Sulfatase-2 from Cancer Associated Fibroblasts: An Environmental Target for Hepatocellular Carcinoma?
Abstract
Introduction: Heparin sulphate proteoglycans in the liver tumour microenvironment (TME) are key regulators of cell signalling, modulated by sulfatase-2 (SULF2). SULF2 overexpression occurs in hepatocellular carcinoma (HCC). Our aims were to define the nature and impact of SULF2 in the HCC TME.
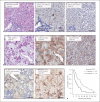
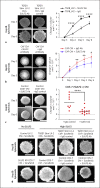
Methods: In liver biopsies from 60 patients with HCC, expression and localization of SULF2 were analysed associated with clinical parameters and outcome. Functional and mechanistic impacts were assessed with immunohistochemistry (IHC), in silico using The Cancer Genome Atlas (TGCA), in primary isolated cancer activated fibroblasts, in monocultures, in 3D spheroids, and in an independent cohort of 20 patients referred for sorafenib. IHC targets included αSMA, glypican-3, β-catenin, RelA-P-ser536, CD4, CD8, CD66b, CD45, CD68, and CD163. SULF2 impact of peripheral blood mononuclear cells was assessed by migration assays, with characterization of immune cell phenotype using fluorescent activated cell sorting.
Results: We report that while SULF2 was expressed in tumour cells in 15% (9/60) of cases, associated with advanced tumour stage and type 2 diabetes, SULF2 was more commonly expressed in cancer-associated fibroblasts (CAFs) (52%) and independently associated with shorter survival (7.2 vs. 29.2 months, p = 0.003). Stromal SULF2 modulated glypican-3/β-catenin signalling in vitro, although in vivo associations suggested additional mechanisms underlying the CAF-SULF2 impact on prognosis. Stromal SULF2 was released by CAFS isolated from human HCC. It was induced by TGFβ1, promoted HCC proliferation and sorafenib resistance, with CAF-SULF2 linked to TGFβ1 and immune exhaustion in TGCA HCC patients. Autocrine activation of PDGFRβ/STAT3 signalling was evident in stromal cells, with the release of the potent monocyte/macrophage chemoattractant CCL2 in vitro. In human PBMCs, SULF2 preferentially induced the migration of macrophage precursors (monocytes), inducing a phenotypic change consistent with immune exhaustion. In human HCC tissues, CAF-SULF2 was associated with increased macrophage recruitment, with tumouroid studies showing stromal-derived SULF2-induced paracrine activation of the IKKβ/NF-κB pathway, tumour cell proliferation, invasion, and sorafenib resistance.
Conclusion: SULF2 derived from CAFs modulates glypican-3/β-catenin signalling but also the HCC immune TME, associated with tumour progression and therapy resistance via activation of the TAK1/IKKβ/NF-κB pathway. It is an attractive target for combination therapies for patients with HCC.
Keywords: Biomarkers; Glypican-3; Hepatocellular carcinoma; Inflammation; Metabolic disease; Molecular targets; NASH; Spheroids; Sulfatase-2; Tumour microenvironment.
Copyright © 2022 by The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
F.O. is a director and shareholder in Fibrofind Ltd. J.L. is a shareholder in Fibrofind Ltd, all other authors declare no competing interests.
Figures




References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68((6)):394–424. - PubMed
-
- Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391((10127)):1301–14. - PubMed
-
- Reeves HL, Zaki MYW, Day CP. Hepatocellular carcinoma in obesity, type 2 diabetes, and NAFLD. Dig Dis Sci. 2016;61((5)):1234–45. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

