A new model for fatty acid hydroxylase-associated neurodegeneration reveals mitochondrial and autophagy abnormalities
- PMID: 36589738
- PMCID: PMC9794614
- DOI: 10.3389/fcell.2022.1000553
A new model for fatty acid hydroxylase-associated neurodegeneration reveals mitochondrial and autophagy abnormalities
Abstract
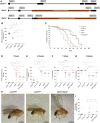
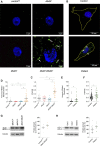
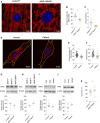
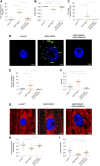
Fatty acid hydroxylase-associated neurodegeneration (FAHN) is a rare disease that exhibits brain modifications and motor dysfunctions in early childhood. The condition is caused by a homozygous or compound heterozygous mutation in fatty acid 2 hydroxylase (FA2H), whose encoded protein synthesizes 2-hydroxysphingolipids and 2-hydroxyglycosphingolipids and is therefore involved in sphingolipid metabolism. A few FAHN model organisms have already been established and give the first insight into symptomatic effects. However, they fail to establish the underlying cellular mechanism of FAHN so far. Drosophila is an excellent model for many neurodegenerative disorders; hence, here, we have characterized and validated the first FAHN Drosophila model. The investigation of loss of dfa2h lines revealed behavioral abnormalities, including motor impairment and flying disability, in addition to a shortened lifespan. Furthermore, alterations in mitochondrial dynamics, and autophagy were identified. Analyses of patient-derived fibroblasts, and rescue experiments with human FA2H, indicated that these defects are evolutionarily conserved. We thus present a FAHN Drosophila model organism that provides new insights into the cellular mechanism of FAHN.
Keywords: Drosophila melanogaster; FA2H; autophagy; fatty acid hydroxylase-associated neurodegeneration; mitochondria.
Copyright © 2022 Mandik, Kanana, Rody, Misera, Wilken, Laabs von Holt, Klein and Vos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

