Sustained therapeutic effect of an anti-inflammatory peptide encapsulated in nanoparticles on ocular vascular leakage in diabetic retinopathy
- PMID: 36589744
- PMCID: PMC9802579
- DOI: 10.3389/fcell.2022.1049678
Sustained therapeutic effect of an anti-inflammatory peptide encapsulated in nanoparticles on ocular vascular leakage in diabetic retinopathy
Abstract
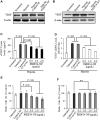
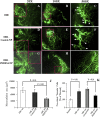
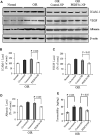
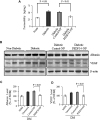
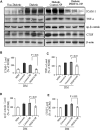
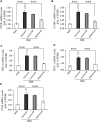
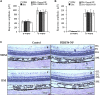
Pigment epithelium-derived factor (PEDF), an endogenous Wnt signaling inhibitor in the serine proteinase inhibitors (SERPIN) super family, is present in multiple organs, including the vitreous. Significantly low levels of PEDF in the vitreous are found to associate with pathological retinal vascular leakage and inflammation in diabetic retinopathy (DR). Intravitreal delivery of PEDF represents a promising therapeutic approach for DR. However, PEDF has a short half-life after intravitreal injection, which represents a major hurdle for the long-term treatment. Here we report the prolonged therapeutic effects of a 34-mer peptide of the PEDF N-terminus, encapsulated in poly (lactic-co-glycolic acid) (PLGA) nanoparticles (PEDF34-NP), on DR. PEDF34-NP inhibited hypoxia-induced expression of vascular endothelial growth factor and reduced levels of intercellular adhesion molecule 1 (ICAM-1) in cultured retinal cells. In addition, PEDF34-NP significantly ameliorated ischemia-induced retinal neovascularization in the oxygen-induced retinopathy rat model, and significantly reduced retinal vascular leakage and inflammation in streptozotocin-induced diabetic rats up to 4 weeks after intravitreal injection, as compared to PLGA-NP control. Intravitreal injection of PEDF34-NP did not display any detectable toxicities to retinal structure and function. Our findings suggest that PEDF34-NP can confer sustained therapeutic effects on retinal inflammation and vascular leakage, having considerable potential to provide long-term treatment options for DR.
Keywords: PLGA; anti-inflammatory; anti-vascular leakage; diabetic retinopathy; nanoparticles; pigment epithelium-derived factor; wnt signaling.
Copyright © 2022 Qu, Park, Zhou, Wassel, Farjo, Criswell, Ma and Zhang.
Conflict of interest statement
DW, RF, was employed by EyeCro LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Adamis A. P., Shima D. T., Tolentino M. J., Gragoudas E. S., Ferrara N., Folkman J., et al. (1996). Inhibition of vascular endothelial growth factor prevents retinal ischemia-associated iris neovascularization in a nonhuman primate. Arch. Ophthalmol. 114, 66–71. 10.1001/archopht.1996.01100130062010 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

