Inverse Design of Pore Wall Chemistry To Control Solute Transport and Selectivity
- PMID: 36589891
- PMCID: PMC9801506
- DOI: 10.1021/acscentsci.2c01011
Inverse Design of Pore Wall Chemistry To Control Solute Transport and Selectivity
Abstract
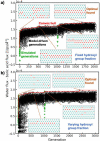
Next-generation membranes for purification and reuse of highly contaminated water require materials with precisely tuned functionality to address key challenges, including the removal of small, charge-neutral solutes. Bioinspired multifunctional membrane surfaces enhance transport properties, but the combinatorically large chemical space is difficult to navigate through trial and error. Here, we demonstrate a computational inverse design approach to efficiently identify promising materials and elucidate design rules. We develop a combined evolutionary optimization, machine learning, and molecular simulation workflow to spatially design chemical functional group patterning in a model nanopore that enhances transport of water relative to solutes. The genetic optimization discovers nonintuitive functionalization strategies that hinder the transport of solutes through the pore, simply by patterning hydrophobic methyl and hydrophilic hydroxyl functional groups. Examining these patterns, we demonstrate that they exploit an unexpected diffusive solute hopping mechanism. This inverse design procedure and the identification of novel molecular mechanisms for pore chemical heterogeneity to impact solute selectivity demonstrate new routes to the design of membrane materials with novel functionalities. More broadly, this work illustrates how chemical design is a powerful strategy to modulate water-mediated surface-solute interactions in complex, soft material systems that are relevant to diverse technologies.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Austin N. D.; Sahinidis N. V.; Trahan D. W. Computer-Aided Molecular Design: An Introduction and Review of Tools, Applications, and Solution Techniques.. Chem. Eng. Res. Des. 2016, 116, 2–26. 10.1016/j.cherd.2016.10.014. - DOI
-
- Monroe J. I.; Jiao S.; Davis R. J.; Robinson Brown D.; Katz L. E.; Shell M. S. Affinity of Small-Molecule Solutes to Hydrophobic, Hydrophilic, and Chemically Patterned Interfaces in Aqueous Solution.. Proc. Natl. Acad. Sci. U. S. A. 2021, 118 (1), e2020205118. 10.1073/pnas.2020205118. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

