Fat infiltration in the infarcted heart as a paradigm for ventricular arrhythmias
- PMID: 36589896
- PMCID: PMC9802586
- DOI: 10.1038/s44161-022-00133-6
Fat infiltration in the infarcted heart as a paradigm for ventricular arrhythmias
Abstract
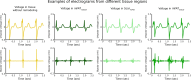
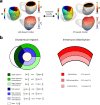
Infiltrating adipose tissue (inFAT) has been recently found to co-localize with scar in infarcted hearts and may contribute to ventricular arrhythmias (VAs), a life-threatening heart rhythm disorder. However, the contribution of inFAT to VA has not been well-established. We investigated the role of inFAT versus scar in VA through a combined prospective clinical and mechanistic computational study. Using personalized computational heart models and comparing the results from simulations of VA dynamics with measured electrophysiological abnormalities during the clinical procedure, we demonstrate that inFAT, rather than scar, is a primary driver of arrhythmogenic propensity and is frequently present in critical regions of the VA circuit. We determined that, within the VA circuitry, inFAT, as opposed to scar, is primarily responsible for conduction slowing in critical sites, mechanistically promoting VA. Our findings implicate inFAT as a dominant player in infarct-related VA, challenging existing paradigms and opening the door for unexplored anti-arrhythmic strategies.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures














References
Grants and funding
LinkOut - more resources
Full Text Sources
