A colorimetric lateral flow immunoassay based on oriented antibody immobilization for sensitive detection of SARS-CoV-2
- PMID: 36589904
- PMCID: PMC9791791
- DOI: 10.1016/j.snb.2022.133245
A colorimetric lateral flow immunoassay based on oriented antibody immobilization for sensitive detection of SARS-CoV-2
Abstract
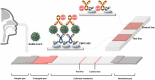
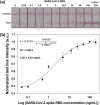
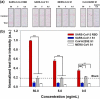
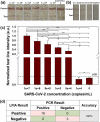
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19). The high human-to-human transmission and rapid evolution of SARS-CoV-2 have resulted in a worldwide pandemic. To contain SARS-CoV-2, it is essential to efficiently control the transmission of the virus through the early diagnosis of infected individuals, including asymptomatic people. Therefore, a rapid and accurate assay is vital for the early diagnosis of SARS-CoV-2 in suspected individuals. In this study, we developed a colorimetric lateral flow immunoassay (LFIA) in which a CBP31-BC linker was used to immobilize antibodies on a cellulose membrane in an oriented manner. The developed LFIA enabled sensitive detection of cultured SARS-CoV-2 in 15 min with a detection limit of 5 × 104 copies/mL. The clinical performance of the LFIA for detecting SARS-CoV-2 was evaluated using 19 clinical samples validated by reverse transcription-polymerase chain reaction (RT-PCR). The LFIA detected all the positive and negative samples accurately, corresponding to 100% accuracy. Importantly, patient samples with low viral loads were accurately identified. Thus, the proposed method can provide a useful platform for rapid and accurate point-of-care testing of SARS-CoV-2 in infected individuals to efficiently control the COVID-19 pandemic.
Keywords: Antibody; COVID-19; Lateral flow immunoassay; Orientation; SARS-CoV-2; Sensitive.
© 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
A novel rapid detection for SARS-CoV-2 spike 1 antigens using human angiotensin converting enzyme 2 (ACE2).Biosens Bioelectron. 2021 Jan 1;171:112715. doi: 10.1016/j.bios.2020.112715. Epub 2020 Oct 15. Biosens Bioelectron. 2021. PMID: 33099241 Free PMC article.
-
Gold nanoparticle conjugate-based lateral flow immunoassay (LFIA) for rapid detection of RBD antigen of SARS-CoV-2 in clinical samples using a smartphone-based application.J Med Virol. 2023 Jan;95(1):e28416. doi: 10.1002/jmv.28416. J Med Virol. 2023. PMID: 36541714 Free PMC article.
-
Next-Generation Rapid and Ultrasensitive Lateral Flow Immunoassay for Detection of SARS-CoV-2 Variants.ACS Sens. 2023 Oct 27;8(10):3733-3743. doi: 10.1021/acssensors.3c01019. Epub 2023 Sep 7. ACS Sens. 2023. PMID: 37675933 Clinical Trial.
-
Development and Efficacy of Lateral Flow Point-of-Care Testing Devices for Rapid and Mass COVID-19 Diagnosis by the Detections of SARS-CoV-2 Antigen and Anti-SARS-CoV-2 Antibodies.Diagnostics (Basel). 2021 Sep 24;11(10):1760. doi: 10.3390/diagnostics11101760. Diagnostics (Basel). 2021. PMID: 34679458 Free PMC article. Review.
-
SERS Based Lateral Flow Immunoassay for Point-of-Care Detection of SARS-CoV-2 in Clinical Samples.ACS Appl Bio Mater. 2021 Apr 19;4(4):2974-2995. doi: 10.1021/acsabm.1c00102. Epub 2021 Mar 17. ACS Appl Bio Mater. 2021. PMID: 35014387 Review.
Cited by
-
Fabrication of natural enzyme-covered / amino-modified Pd-Pt bimetallic-doped zeolitic imidazolate framework for ultrasensitive detection of metabolites.Anal Sci. 2025 Jan;41(1):23-34. doi: 10.1007/s44211-024-00670-z. Epub 2024 Oct 3. Anal Sci. 2025. PMID: 39363137
-
A semi-quantitative upconversion nanoparticle-based immunochromatographic assay for SARS-CoV-2 antigen detection.Front Microbiol. 2023 Dec 11;14:1289682. doi: 10.3389/fmicb.2023.1289682. eCollection 2023. Front Microbiol. 2023. PMID: 38149276 Free PMC article.
-
Lateral Flow Assay: A Summary of Recent Progress for Improving Assay Performance.Biosensors (Basel). 2023 Aug 23;13(9):837. doi: 10.3390/bios13090837. Biosensors (Basel). 2023. PMID: 37754072 Free PMC article. Review.
-
Recent Advances in Lateral Flow Assays for Viral Protein Detection with Nanomaterial-Based Optical Sensors.Biosensors (Basel). 2024 Apr 17;14(4):197. doi: 10.3390/bios14040197. Biosensors (Basel). 2024. PMID: 38667190 Free PMC article. Review.
-
The Evaluation of a Lateral Flow Strip Based on the Covalently Fixed "End-On" Orientation of an Antibody for Listeria monocytogenes Detection.Anal Chem. 2024 May 28;96(21):8543-8551. doi: 10.1021/acs.analchem.4c00533. Epub 2024 May 15. Anal Chem. 2024. PMID: 38748432 Free PMC article.
References
-
- W.H.O., Weekly epidemiological update on COVID-19 - 28 December 2021 (2021).
-
- Baker A.N., Richards S.J., Guy C.S., Congdon T.R., Hasan M., Zwetsloot A.J., Gallo A., Lewandowski J.R., Stansfeld P.J., Straube A., Walker M., Chessa S., Pergolizzi G., Dedola S., Field R.A., Gibson M.I. The SARS-COV-2 spike protein binds sialic acids and enables rapid detection in a lateral flow point of care diagnostic device. ACS Cent. Sci. 2020;6(11):2046–2052. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

