Neonatal intake of Omega-3 fatty acids enhances lipid oxidation in adipocyte precursors
- PMID: 36590177
- PMCID: PMC9800552
- DOI: 10.1016/j.isci.2022.105750
Neonatal intake of Omega-3 fatty acids enhances lipid oxidation in adipocyte precursors
Abstract
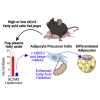
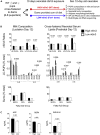
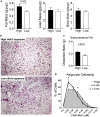
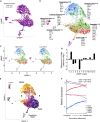
Establishing metabolic programming begins during fetal and postnatal development, and early-life lipid exposures play a critical role during neonatal adipogenesis. We define how neonatal consumption of a low omega-6 to -3 fatty acid ratio (n6/n3 FA ratio) establishes FA oxidation in adipocyte precursor cells (APCs) before they become adipocytes. In vivo, APCs isolated from mouse pups exposed to the low n6/n3 FA ratio had superior FA oxidation capacity, elevated beige adipocyte mRNAs Ppargc1α, Ucp2, and Runx1, and increased nuclear receptor NR2F2 protein. In vitro, APC treatment with NR2F2 ligand-induced beige adipocyte mRNAs and increased mitochondrial potential but not mass. Single-cell RNA-sequencing analysis revealed low n6/n3 FA ratio yielded more mitochondrial-high APCs and linked APC NR2F2 levels with beige adipocyte signatures and FA oxidation. Establishing beige adipogenesis is of clinical relevance, because fat depots with energetically active, smaller, and more numerous adipocytes improve metabolism and delay metabolic dysfunction.
Keywords: Biological sciences; Endocrine regulation; Endocrinology.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

