Green synthesis of multifunctional MgO@AgO/Ag2O nanocomposite for photocatalytic degradation of methylene blue and toluidine blue
- PMID: 36590276
- PMCID: PMC9798100
- DOI: 10.3389/fchem.2022.1083596
Green synthesis of multifunctional MgO@AgO/Ag2O nanocomposite for photocatalytic degradation of methylene blue and toluidine blue
Abstract
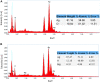
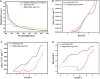
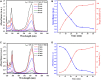
Introduction: In this paper, MgO@AgO/Ag2O nanoparticles were greenly synthesized, the current idea is to replace the harmful chemical technique with an ecofriendly synthesis of metal oxide nanoparticles (NPs) utilizing biogenic sources. Methods: The current investigation was conducted to create silver oxide NPs decorated by MgO NPs (namely, MgO@AgO/Ag2O nanocom-posite) using the leaves extract of Purslane (Portulaca Oleracea) as the reducing and capping agent. The nanopowder was investigated by means of X-ray diffraction, scanning electron mi-croscope, BET surface area, Fourier transform infrared, and UV-vis spectrophotom-eter studies. XRD studies reveal the monophasic nature of these highly crystalline silver nano-particles. SEM studies the shape and morphology of the synthesis AgO/Ag2O and MgO@AgO/Ag2O NPs. The presence of magnesium and oxygen was further confirmed by EDS profile. Results and discussion: The surface area was found to be 9.1787 m2/g and 7.7166 m2/g, respectively. FTIR analysis showed the presence of specific functional groups. UV-vis spectrophotometer studies show the absorption band at 450 nm due to surface plasmon resonance. The results have also indicated the high performance of the greenly synthesized AgO/Ag2O NPs and MgO@AgO/Ag2O NPs for photocatalytic activity dye degradation (methylene blue and toluidine blue).
Keywords: MgO@AgO/Ag2O NPs; Portulaca oleracea; green synthesis; methylene blue; photocatalysis; toluidine blue.
Copyright © 2022 Zidane, Laouini, Bouafia, Meneceur, Tedjani, Alshareef, Almukhlifi, Al-Essa, Al-Essa, Rahman, Madkhali and Menaa.
Conflict of interest statement
Author FM was employed by the company Fluorotronics, Inc.-California Innovations Corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Ahmad M., Rehman W., Khan M. M., Qureshi M. T., Gul A., Haq S., et al. (2021). Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of Rhodamine B. J. Environ. Chem. Eng. 9, 104725. 10.1016/j.jece.2020.104725 - DOI
-
- Abdullah J. A. A., Salah Eddine L., Abderrhmane B., Alonso-González M., Guerrero A., Romero A. (2020). Green synthesis and characterization of iron oxide nanoparticles by pheonix dactylifera leaf extract and evaluation of their antioxidant activity. Sustain. Chem. Pharm. 17, 100280. 10.1016/j.scp.2020.100280 - DOI
-
- Ahmadi O., Jafarizadeh-Malmiri H., Jodeiri N. J. G. P., Synthesis (2018). Eco-friendly microwave-enhanced green synthesis of silver nanoparticles using Aloe vera leaf extract and their physico-chemical and antibacterial studies. Green Process. Synthesis 7, 231–240. 10.1515/gps-2017-0039 - DOI
-
- Aisida S. O., Ugwu K., Akpa P. A., Nwanya A. C., Nwankwo U., Botha S. S., et al. (2019). Biosynthesis of silver nanoparticles using bitter leave (Veronica amygdalina) for antibacterial activities. Surfaces Interfaces 17, 100359. 10.1016/j.surfin.2019.100359 - DOI
-
- Al Essa K., Radha A., Navrotsky A. (2021). “Drop solution calorimetric studies of interface enthalpy of cubic silver (I) oxide (Ag2O) nanocrystals,” in Proceedings of the key engineering materials, 73.
LinkOut - more resources
Full Text Sources

