Linking the gut microbiome to microglial activation in opioid use disorder
- PMID: 36590299
- PMCID: PMC9800800
- DOI: 10.3389/fnins.2022.1050661
Linking the gut microbiome to microglial activation in opioid use disorder
Abstract
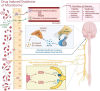
Substance use disorder (SUD) is a physical and psychological disorder globally prevalent today that has resulted in over 107,000 drug overdose deaths in 2021 in the United States alone. This manuscript reviews the potential relationship between opioid use disorder (OUD), a prevalent subset of SUD, and the microglia, the resident macrophages of the central nervous system (CNS), as they have been found to become significantly more activated during opioid exposure. The inflammatory response mediated by the microglia could contribute to the pathophysiology of SUDs, in particular OUD. Further understanding of the microglia and how they respond to not only signals in the CNS but also signals from other areas of the body, such as the gut microbiome, could explain how the microglia are involved in drug use. Several studies have shown extensive communication between the gut microbiome and the microglia, which may be an important factor in the initiation and development of OUD. Particularly, strategies seeking to manipulate and restore the gut microbiome have been shown to reduce microglial activation and attenuate inflammation. In this review, we discuss the evidence for a link between the microglia and OUD and how the gut microbiome might influence microglial activation to drive the disorder and its associated behaviors. Understanding this connection between microglia and the gut microbiome in the context of drug use may present additional therapeutic targets to treat the different stages of drug use.
Keywords: OUD; SUD; microbiome; microglia; opioids.
Copyright © 2022 Antoine, Venigalla, Truitt and Roy.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ahmad F. B., Rossen L. M. (2020). Vital Statistics Rapid Release- Provisional Drug Overdose Data. Atlanta: National Vitals Statistics System.
-
- Akira S., Uematsu S., Takeuchi O. (2006). Pathogen Recognition and Innate Immunity. Cell 124 783–801. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

