Enhanced perceptual task performance without deprivation in mice using medial forebrain bundle stimulation
- PMID: 36590697
- PMCID: PMC9795331
- DOI: 10.1016/j.crmeth.2022.100355
Enhanced perceptual task performance without deprivation in mice using medial forebrain bundle stimulation
Abstract
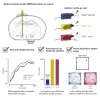
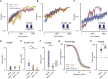
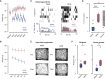
Perceptual decision-making tasks are essential to many fields of neuroscience. Current protocols generally reward deprived animals with water. However, balancing animals' deprivation level with their well-being is challenging, and trial number is limited by satiation. Here, we present electrical stimulation of the medial forebrain bundle (MFB) as an alternative that avoids deprivation while yielding stable motivation for thousands of trials. Using licking or lever press as a report, MFB animals learnt auditory discrimination tasks at similar speed to water-deprived mice. Moreover, they more reliably reached higher accuracy in harder tasks, performing up to 4,500 trials per session without loss of motivation. MFB stimulation did not impact the underlying sensory behavior since psychometric parameters and response times are preserved. MFB mice lacked signs of metabolic or behavioral stress compared with water-deprived mice. Overall, MFB stimulation is a highly promising tool for task learning because it enhances task performance while avoiding deprivation.
Keywords: Go/No-go; audition; behavior; lever pressing; licking; medial forebrain bundle; motivation; mouse; perception; perceptual decision-making; reinforcement learning; satiation.
© 2022 The Authors.
Conflict of interest statement
None of the authors have any competing interests to declare.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

