SARS-CoV-2 seroprevalence in children worldwide: A systematic review and meta-analysis
- PMID: 36590788
- PMCID: PMC9795163
- DOI: 10.1016/j.eclinm.2022.101786
SARS-CoV-2 seroprevalence in children worldwide: A systematic review and meta-analysis
Abstract
Background: The higher hospitalisation rates of those aged 0-19 years (referred to herein as 'children') observed since the emergence of the immune-evasive SARS-CoV-2 Omicron variant and subvariants, along with the persisting vaccination disparities highlighted a need for in-depth knowledge of SARS-CoV-2 sero-epidemiology in children. Here, we conducted this systematic review to assess SARS-CoV-2 seroprevalence and determinants in children worldwide.
Methods: In this systematic review and meta-analysis study, we searched international and preprinted scientific databases from December 1, 2019 to July 10, 2022. Pooled seroprevalences were estimated according to World Health Organization (WHO) regions (at 95% confidence intervals, CIs) using random-effects meta-analyses. Associations with SARS-CoV-2 seroprevalence and sources of heterogeneity were investigated using sub-group and meta-regression analyses. The protocol used in this study has been registered in PROSPERO (CRD42022350833).
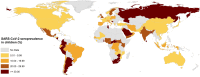
Findings: We included 247 studies involving 757,075 children from 70 countries. Seroprevalence estimates varied from 7.3% (5.8-9.1%) in the first wave of the COVID-19 pandemic to 37.6% (18.1-59.4%) in the fifth wave and 56.6% (52.8-60.5%) in the sixth wave. The highest seroprevalences in different pandemic waves were estimated for South-East Asia (17.9-81.8%) and African (17.2-66.1%) regions; while the lowest seroprevalence was estimated for the Western Pacific region (0.01-1.01%). Seroprevalence estimates were higher in children at older ages, in those living in underprivileged countries or regions, and in those of minority ethnic backgrounds.
Interpretation: Our findings indicate that, by the end of 2021 and before the Omicron wave, around 50-70% of children globally were still susceptible to SARS-CoV-2 infection, clearly emphasising the need for more effective vaccines and better vaccination coverage among children and adolescents, particularly in developing countries and minority ethnic groups.
Funding: None.
Keywords: COVID-19; Children; Meta-analysis; SARS-CoV-2; Seroprevalence; Serum antibodies.
© 2022 The Author(s).
Conflict of interest statement
All authors declare no competing interests. Prof. Hotez is a co-inventor of a COVID-19 recombinant protein vaccine technology owned by Baylor College of Medicine (BCM) that was recently licensed by Baylor Ventures non-exclusively and with no patent restrictions to several companies committed to advance vaccines for low- and middle-income countries. These include Biological E (India–CORBEVAX) and BioFarma (Indonesia–INDOVAC), Incepta (Bangladesh). He has no involvement in license negotiations conducted by BCM. Similar to other research universities, a long-standing BCM policy provides its faculty and staff, who make discoveries and that result in a commercial license, a share of any royalty income. To date, BCM has not distributed any royalty income to the co-inventors on the COVID-19 recombinant protein vaccine technology.
Figures


References
-
- World Health Organisation WHO weekly epidemiological update on Covid-19—16 November 2022. 2022. https://www.who.int/publications/m/item/weekly-epidemiological-update-on...
LinkOut - more resources
Full Text Sources
Miscellaneous

