Real world experience with ropeginterferon alpha-2b (Besremi) in essential thrombocythaemia and polycythaemia vera following exposure to pegylated interferon alfa-2a (Pegasys)
- PMID: 36590864
- PMCID: PMC9801096
- DOI: 10.1016/j.lrr.2022.100360
Real world experience with ropeginterferon alpha-2b (Besremi) in essential thrombocythaemia and polycythaemia vera following exposure to pegylated interferon alfa-2a (Pegasys)
Abstract
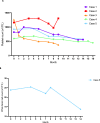
Despite widespread use of Pegylated forms of Inteferon in the management of Myeloproliferative Neoplasms (MPN), most clinicians have experience predominantly with peginterferon alfa-2a (Pegasys). Third generation pegylated IFNα, ropeginterferon alfa-2b (ropegIFN; Besremi), was recommended by the European Medicine Authority (EMA) for treatment of Polycythaemia Vera (PV) following a Phase III trial (PROUD-PV / CONTINUATION-PV). FDA approval for PV, regardless of treatment history, was subsequently granted in November 2021. We hereby demonstrate the safety and tolerability of ropegIFN in a series of MPN patients at variable doses. It corroborates reports of efficacy of ropegIFN in patients with PV and use in pregnancy.
Keywords: Essential Thrombocythaemia; IFN; MPN; Polycythaemia Vera.
© 2022 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
Patrick Harrington: research funding from Bristol Myers Squibb, Honoraria and 10.13039/100017655Incyte. Claire N. Harrison: Advisory board and speaker fees: AOP and Roche.
Figures
References
-
- Gisslinger Heinz, Klade Christoph, Georgiev Pencho, Krochmalczyk Dorota, Gercheva-Kyuchukova Liana, Egyed Miklos, Dulicek Petr, Illés Árpád, Pylypenko Halyna, Sivcheva Lylia, Jiří Mayer11 P. Long-term use of ropeginterferon alpha-2b in polycythemia vera: 5-year results from a randomized controlled study and its extension. Am. Soc. Haematol. 2020 https://ash.confex.com/ash/2020/webprogram/Paper136973.html MD1*PhD2*MD3*MD4*MD5*MD6*MD7*MDPhD8*MD9*MD10* Published.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials