Discovery of imidazole-based GSK-3 β inhibitors for transdifferentiation of human mesenchymal stem cells to neurons: A potential single-molecule neurotherapeutic foresight
- PMID: 36590911
- PMCID: PMC9797524
- DOI: 10.3389/fnmol.2022.1002419
Discovery of imidazole-based GSK-3 β inhibitors for transdifferentiation of human mesenchymal stem cells to neurons: A potential single-molecule neurotherapeutic foresight
Abstract
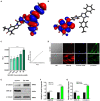
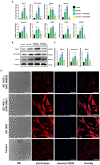
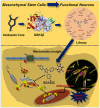
The transdifferentiation of human mesenchymal stem cells (hMSC) to functional neurons is crucial for the development of future neuro-regenerative therapeutics. Currently, transdifferentiation of hMSCs to neurons requires a "chemical cocktail" along with neural growth factors. The role of the individual molecules present in a "chemical cocktail" is poorly understood and may cause unwanted toxicity or adverse effects. Toward, this goal, we have showcased the discovery of an imidazole-based "single-molecule" transdifferentiation initiator SG-145C. This discovery was achieved via screening of a small molecule library through extensive in silico studies to shortlist the best-fitting molecules. This discovery evolved through a careful selection to target Glycogen synthase kinase-3β (GSK-3β), which is one of the important proteins responsible for neurogenesis. Rigorous computational experiments, as well as extensive biological assays, confirmed that SG-145C has significant potential to transdifferentiate hMSCs to neurons. Interestingly, our results suggest that SG-145C can inhibit the proteasomal degradation of phosphorylated β-catenin, in turn promoting transdifferentiation of hMSCs into neurons via the Wnt pathway.
Keywords: GSK-3β; hMSC; imidazole; neurodegeneration; transdifferentiation; β-III tubulin; β-catenin.
Copyright © 2022 Gupta, Mahata, Roy, Gharai, Jana, Garg and Ghosh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

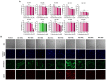
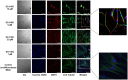
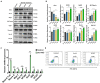
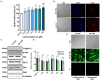
Similar articles
-
Brain-derived Neurotrophic Factor Promotes Growth of Neurons and Neural Stem Cells Possibly by Triggering the Phosphoinositide 3-Kinase/ AKT/Glycogen Synthase Kinase-3β/β-catenin Pathway.CNS Neurol Disord Drug Targets. 2017;16(7):828-836. doi: 10.2174/1871527316666170518170422. CNS Neurol Disord Drug Targets. 2017. PMID: 28524001
-
Lithium stimulates human bone marrow derived mesenchymal stem cell proliferation through GSK-3β-dependent β-catenin/Wnt pathway activation.FEBS J. 2014 Dec;281(23):5371-89. doi: 10.1111/febs.13081. Epub 2014 Oct 27. FEBS J. 2014. PMID: 25265417
-
Extracellular matrix stiffness controls osteogenic differentiation of mesenchymal stem cells mediated by integrin α5.Stem Cell Res Ther. 2018 Mar 1;9(1):52. doi: 10.1186/s13287-018-0798-0. Stem Cell Res Ther. 2018. PMID: 29490668 Free PMC article.
-
Drug development targeting the glycogen synthase kinase-3beta (GSK-3beta)-mediated signal transduction pathway: inhibitors of the Wnt/beta-catenin signaling pathway as novel anticancer drugs.J Pharmacol Sci. 2009 Feb;109(2):179-83. doi: 10.1254/jphs.08r28fm. Epub 2009 Jan 29. J Pharmacol Sci. 2009. PMID: 19179804 Review.
-
Lithium and neuropsychiatric therapeutics: neuroplasticity via glycogen synthase kinase-3beta, beta-catenin, and neurotrophin cascades.J Pharmacol Sci. 2009 May;110(1):14-28. doi: 10.1254/jphs.09r02cr. Epub 2009 May 8. J Pharmacol Sci. 2009. PMID: 19423950 Review.
Cited by
-
Can Oxytocin Neuropeptide Promote Human Mesenchymal Stem Cells to Neuron Conversion?: A Novel Approach in Future Neurotherapeutic Research.ACS Chem Neurosci. 2025 Aug 6;16(15):2753-2755. doi: 10.1021/acschemneuro.5c00520. Epub 2025 Jul 17. ACS Chem Neurosci. 2025. PMID: 40671480 Free PMC article. Review.
-
Gallic Acid Alleviates Cognitive Impairment by Promoting Neurogenesis via the GSK3β-Nrf2 Signaling Pathway in an APP/PS1 Mouse Model.J Alzheimers Dis Rep. 2024 Mar 15;8(1):461-477. doi: 10.3233/ADR-230171. eCollection 2024. J Alzheimers Dis Rep. 2024. PMID: 38549642 Free PMC article.
-
Dual-Function Fluorescent Probes for Neuronal Trans-Differentiation: A Promising Therapeutic Strategy in Neuroregenerative Research.ACS Chem Neurosci. 2025 Jul 16;16(14):2561-2563. doi: 10.1021/acschemneuro.5c00367. Epub 2025 Jun 25. ACS Chem Neurosci. 2025. PMID: 40560137 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

