International oncology drug approvals for multiregional or single-country clinical trials: A systematic review
- PMID: 36590932
- PMCID: PMC9798114
- DOI: 10.3389/fmed.2022.1084980
International oncology drug approvals for multiregional or single-country clinical trials: A systematic review
Abstract
Background: Cancer remains one of the most common causes of morbidity and mortality worldwide. Multiregional (MRCTs) and single-country clinical trials are two common approaches to support new oncology drug approvals internationally. However, systematic reviews comparing MRCTs with single-country trials for international oncology drug approval are lacking.
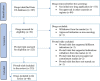
Methods: We searched health agency websites to retrieve all approved oncology drugs from 2010 to 2022. ClinicalTrials.gov was used to retrieve all pivotal study information. We used an adapted version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2) and Risk Of Bias In Non-randomized Studies - of Interventions (ROBINS-I) checklist to assess the risk-of-bias in randomized and non-randomized trials, respectively.
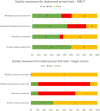
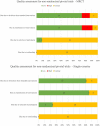
Results: A total of 48 new drugs and biologics (comprising 215 pivotal clinical trials) with initial marketing approval in the United States, European Union, Japan, and China were included. The reporting quality of MRCTs vs. single-country studies was similar. The median time interval for approval was significantly longer for MRCTs than for single-country bridging studies (1,399 vs. 975 days, P < 0.0001), whereas the median time interval for approval was shorter for MRCTs than for single-country standalone studies. The time gap for oncology drugs approved before 2015 was significantly longer than for those approved after 2015. The median timeline for approval in MRCTs involving 3 regions showed the shortest time-to-approval compared with MRCTs involving 4-5 and 1-2 regions. There was no significant difference in the time-to-approval among different tumor types and product types.
Conclusion: The median time-to-approval of MRCTs was significantly longer than that of single-country bridging studies but shorter than that of single-country standalone studies, primarily involving 3 regions as the most frequent pattern and the shortest time-to-approval to operate MRCTs as a pivotal trial. Single-country bridging studies still provide essential supplements for international oncology drug approvals if MRCTs do not apply. Future studies should explore how to shorten the time-to-approval for MRCTs.
Systematic review registration: [https://www.researchregistry.com/browsethe-registry#registryofsystematicreviewsmeta-analyses/], identifier [1390].
Keywords: MRCTs; international; oncology drug approvals; single-country; time-to-approval.
Copyright © 2022 Zhang, Onakpoya and Rupalla.
Conflict of interest statement
KR was employed by Widler & Schiemann AG and Ymmunobio AG. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- World Cancer Research Fund International [WCRF]. Worldwide cancer data. (2020). Available online at: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/ (accessed July 27, 2022).
-
- Albrecht B, Alfano S, Keane H, Yang G. Delivering innovation: 2020 oncology market outlook. McKinsey & Company. (2021). Available online at: https://www.mckinsey.com/industries/life-sciences/our-insights/deliverin... (accessed July 27, 2022).
-
- IQVIA. Global oncology trends. (2022). Available online at: https://www.iqvia.com/insights/the-iqvia-institute/reports/global-oncolo... (accessed July 27, 2022).
-
- Food and Drug Administration [FDA]. (2022). CFR - Code of federal regulations title 21. Available online at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?f... (accessed July 27, 2022).
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous