Visible-Light-Driven Solventylation Strategy for Olefin Functionalization
- PMID: 36591128
- PMCID: PMC9798500
- DOI: 10.1021/acsomega.2c07172
Visible-Light-Driven Solventylation Strategy for Olefin Functionalization
Abstract
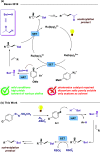
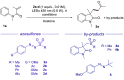
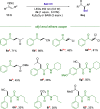
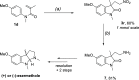
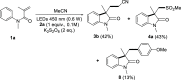
Amphiphilic aryl radicals generated upon visible light irradiation of arylazo sulfones have been exploited in the development of a solventylation strategy via hydrogen atom transfer (HAT). The present protocol succeeded in the versatile functionalization of various olefins with carbon-centered radicals deriving from acetone, acetonitrile, chloroform, methylene chloride, nitromethane, methyl acetate, and methyl formate under metal- and photocatalyst-free conditions. The direct addition of the aryl radicals onto the olefin substrates was suppressed under high dilution conditions.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
-
See for review:
- Guillemard L.; Kaplaneris N.; Ackermann L.; Johansson M. J. Late-stage C–H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 2021, 5, 522–545. 10.1038/s41570-021-00300-6. - DOI - PubMed
- Börgel J.; Ritter T. Late-stage functionalization. Chem 2020, 6, 1877–1887. 10.1016/j.chempr.2020.07.007. - DOI
- Baudoin O. Multiple catalytic C–H Bond Functionalization for Natural Product Synthesis. Angew. Chem., Int. Ed. 2020, 59, 17798–17809. 10.1002/anie.202001224. - DOI - PubMed
-
-
-
See for review:
- Capaldo L.; Quadri L.; Ravelli D. Photocatalytic hydrogen atom transfer: the philosopher’s stone for late-stage functionalization?. Green Chem. 2020, 22, 3376–3396. 10.1039/D0CS00493F. - DOI
- Cannalire R.; Pelliccia S.; Sancineto L.; Novellino E.; Tron G. C.; Giustiniano M. Visible light photocatalysis in the late-stage functionalization of pharmaceutically relevant compounds. Chem. Soc. Rev. 2021, 50, 766–897. 10.1039/D0CS00493F. - DOI - PubMed
-
-
- Murray P. R. D.; Cox J. H.; Chiappini D. N.; Roos C. B.; McLoughlin E. A.; Hejna B. G.; Nguyen S. T.; Ripberger H. H.; Ganley J. M.; Tsui E.; Shin N. Y.; Koronkiewicz B.; Qiu G.; Knowles R. R. Photochemical and Electrochemical Applications of Proton-Coupled Electron Transfer in Organic Synthesis. Chem. Rev. 2022, 122, 2017–2291. 10.1021/acs.chemrev.1c00374. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

