Polymerization and Applications of Poly(methyl methacrylate)-Graphene Oxide Nanocomposites: A Review
- PMID: 36591191
- PMCID: PMC9798503
- DOI: 10.1021/acsomega.2c04483
Polymerization and Applications of Poly(methyl methacrylate)-Graphene Oxide Nanocomposites: A Review
Abstract
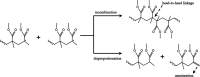
Graphene oxide (GO)-incorporated poly(methyl methacrylate) (PMMA) nanocomposites (PMMA-GO) have demonstrated a wide range of outstanding mechanical, electrical, and physical characteristics. It is of interest to review the synthesis of PMMA-GO nanocomposites and their applications as multifunctional structural materials. The attention of this review is to focus on the radical polymerization techniques, mainly bulk and emulsion polymerization, to prepare PMMA-GO polymeric nanocomposite materials. This review also discusses the effect of solvent polarity on the polymerization process and the types of surfactants (anionic, cationic, nonionic) and initiator used in the polymerization. PMMA-GO nanocomposite synthesis using radical polymerization-based techniques is an active topic of study with several prospects for considerable future improvement and a variety of possible emerging applications. The concentration and dispersity of GO used in the polymerization play critical roles to ensure the functionality and performance of the PMMA-GO nanocomposites.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Zhang W.; Müller A. H. E. Architecture, Self-Assembly and Properties of Well-Defined Hybrid Polymers Based on Polyhedral Oligomeric Silsequioxane (POSS. Prog. Polym. Sci. 2013, 38 (8), 1121–1162. 10.1016/j.progpolymsci.2013.03.002. - DOI
-
- Swolfs Y.; Gorbatikh L.; Verpoest I. Fibre Hybridisation in Polymer Composites: A Review. Compos. Part A Appl. Sci. Manuf. 2014, 67, 181–200. 10.1016/j.compositesa.2014.08.027. - DOI
-
- Kaur S.; Gallei M.; Ionescu E. Polymer–Ceramic Nanohybrid Materials. Organice-Inorganic Hybrid Nanomaterials; Sprinter International Publishing: Cham 2014, 267, 143–185. 10.1007/12_2014_282. - DOI
-
- Sun J.; Ma Q.; Xue D.; Shan W.; Liu R.; Dong B.; Zhang J.; Wang Z.; Shao B. Polymer/Inorganic Nanohybrids: An Attractive Materials for Analysis and Sensing. TrAC Trends Anal. Chem. 2021, 140, 116273.10.1016/j.trac.2021.116273. - DOI
Publication types
LinkOut - more resources
Full Text Sources

