Oxygen level is a critical regulator of human B cell differentiation and IgG class switch recombination
- PMID: 36591315
- PMCID: PMC9795029
- DOI: 10.3389/fimmu.2022.1082154
Oxygen level is a critical regulator of human B cell differentiation and IgG class switch recombination
Abstract
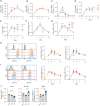
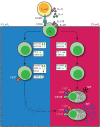
The generation of high-affinity antibodies requires an efficient germinal center (GC) response. As differentiating B cells cycle between GC dark and light zones they encounter different oxygen pressures (pO2). However, it is essentially unknown if and how variations in pO2 affect B cell differentiation, in particular for humans. Using optimized in vitro cultures together with in-depth assessment of B cell phenotype and signaling pathways, we show that oxygen is a critical regulator of human naive B cell differentiation and class switch recombination. Normoxia promotes differentiation into functional antibody secreting cells, while a population of CD27++ B cells was uniquely generated under hypoxia. Moreover, time-dependent transitions between hypoxic and normoxic pO2 during culture - reminiscent of in vivo GC cyclic re-entry - steer different human B cell differentiation trajectories and IgG class switch recombination. Taken together, we identified multiple mechanisms trough which oxygen pressure governs human B cell differentiation.
Keywords: B cells; antibody-secreting cell; class switch recombination; differentiation; germinal center; hypoxia.
Copyright © 2022 Koers, Marsman, Steuten, Tol, Derksen, ten Brinke, van Ham and Rispens.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

