Targeting Epsins to Inhibit Fibroblast Growth Factor Signaling While Potentiating Transforming Growth Factor-β Signaling Constrains Endothelial-to-Mesenchymal Transition in Atherosclerosis
- PMID: 36591786
- PMCID: PMC10136057
- DOI: 10.1161/CIRCULATIONAHA.122.063075
Targeting Epsins to Inhibit Fibroblast Growth Factor Signaling While Potentiating Transforming Growth Factor-β Signaling Constrains Endothelial-to-Mesenchymal Transition in Atherosclerosis
Abstract
Background: Epsin endocytic adaptor proteins are implicated in the progression of atherosclerosis; however, the underlying molecular mechanisms have not yet been fully defined. In this study, we determined how epsins enhance endothelial-to-mesenchymal transition (EndoMT) in atherosclerosis and assessed the efficacy of a therapeutic peptide in a preclinical model of this disease.
Methods: Using single-cell RNA sequencing combined with molecular, cellular, and biochemical analyses, we investigated the role of epsins in stimulating EndoMT using knockout in Apoe-/- and lineage tracing/proprotein convertase subtilisin/kexin type 9 serine protease mutant viral-induced atherosclerotic mouse models. The therapeutic efficacy of a synthetic peptide targeting atherosclerotic plaques was then assessed in Apoe-/- mice.
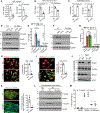
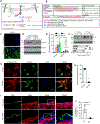
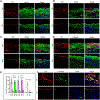
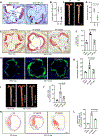
Results: Single-cell RNA sequencing and lineage tracing revealed that epsins 1 and 2 promote EndoMT and that the loss of endothelial epsins inhibits EndoMT marker expression and transforming growth factor-β signaling in vitro and in atherosclerotic mice, which is associated with smaller lesions in the Apoe-/- mouse model. Mechanistically, the loss of endothelial cell epsins results in increased fibroblast growth factor receptor-1 expression, which inhibits transforming growth factor-β signaling and EndoMT. Epsins directly bind ubiquitinated fibroblast growth factor receptor-1 through their ubiquitin-interacting motif, which results in endocytosis and degradation of this receptor complex. Consequently, administration of a synthetic ubiquitin-interacting motif-containing peptide atheroma ubiquitin-interacting motif peptide inhibitor significantly attenuates EndoMT and progression of atherosclerosis.
Conclusions: We conclude that epsins potentiate EndoMT during atherogenesis by increasing transforming growth factor-β signaling through fibroblast growth factor receptor-1 internalization and degradation. Inhibition of EndoMT by reducing epsin-fibroblast growth factor receptor-1 interaction with a therapeutic peptide may represent a novel treatment strategy for atherosclerosis.
Keywords: EndoMT; adaptor proteins, signal transducing; atherosclerosis; endocytosis; epsin; peptides; receptor, fibroblast growth factor, type 1; single-cell gene expression analysis; transforming growth factor beta; vascular diseases.
Figures




References
-
- Linton MRF, Yancey PG, Davies SS, Jerome WG, Linton EF, Song WL, Doran AC and Vickers KC. The Role of Lipids and Lipoproteins in Atherosclerosis. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrere B, Levy M, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, and Wilson DP, eds. Endotext South Dartmouth (MA); 2000.
-
- Hansson GK, Robertson AK and Soderberg-Naucler C. Inflammation and atherosclerosis. Ann Rev Pathol. 2006;1:297–329. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM138407/GM/NIGMS NIH HHS/United States
- R01 HL133216/HL/NHLBI NIH HHS/United States
- R01 HL133254/HL/NHLBI NIH HHS/United States
- R01 HL146134/HL/NHLBI NIH HHS/United States
- R01 HL093242/HL/NHLBI NIH HHS/United States
- R01 HL158097/HL/NHLBI NIH HHS/United States
- R01 HL156362/HL/NHLBI NIH HHS/United States
- R01 HL162367/HL/NHLBI NIH HHS/United States
- R01 HL137229/HL/NHLBI NIH HHS/United States
- R01 HL150106/HL/NHLBI NIH HHS/United States
- I01 BX004426/BX/BLRD VA/United States
- R01 GM125632/GM/NIGMS NIH HHS/United States
- R01 HL118676/HL/NHLBI NIH HHS/United States
- R01 HL130845/HL/NHLBI NIH HHS/United States
- R01 HL141853/HL/NHLBI NIH HHS/United States
- R01 HL148338/HL/NHLBI NIH HHS/United States
- R01 HL167206/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

