Involvement of angiogenesis in cancer-associated acinar-to-ductal metaplasia lesion of pancreatic cancer invasive front
- PMID: 36592214
- PMCID: PMC11797116
- DOI: 10.1007/s00432-022-04554-5
Involvement of angiogenesis in cancer-associated acinar-to-ductal metaplasia lesion of pancreatic cancer invasive front
Abstract
Purpose: This study aimed to demonstrate the involvement of angiogenesis in cancer-associated acinar-to-ductal metaplasia (CA-ADM) lesion of invasive front pancreatic ductal adenocarcinoma (PDAC) and investigate the possible mechanism.
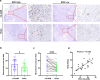
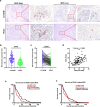
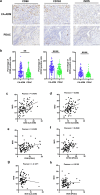
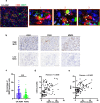
Methods: Tissue samples from 128 patients with PDAC and 36 LSL-KrasG12D/+; LSL-Trp53R172H/+; Pdx-1-Cre mice were analyzed. Immunohistochemical assay was performed using HE, anti-CK19 and anti-amylase to confirm the presence of CA-ADM lesions, using anti-CD34 and anti-CD31 to measure microvessel density (MVD), and using anti-CD68, anti-CD163, anti-iNOS, or anti-MMP9 to evaluate the immune microenvironment. We performed multiplex immunohistochemical assay to detect the co-expression of MMP9 and CD68 on macrophage. We examined clinical outcomes and other clinicopathological factors to determine the significance of high-level MVD of CA-ADM on survival and liver metastasis. We performed tube formation assay to evaluate the effect of macrophage on angiogenic capacity in vitro.
Results: Angiogenesis was significantly abundant in CA-ADM lesions compared with that in PDAC lesions in human and mouse tissues. High-level MVD in CA-ADM lesions was an independent predictor of poor prognosis (P = 0.0047) and the recurrence of liver metastasis (P = 0.0027). More CD68-positive and CD163-positive macrophages were detected in CA-ADM lesions than in PDAC. The percentage of CD68-positive macrophages was positively correlated with MVD in CA-ADM lesions. Multiplex-immunostaining revealed that MMP9 was expressed in CD68-positive macrophages of CA-ADM lesions. In CA-ADM lesions, the percentage of macrophages was positively correlated with MMP9 expression, which positively correlated with microvessel density.
Conclusion: CA-ADM related angiogenesis is a promising predictive marker for poor prognosis of PDAC and may provide an attractive therapeutic target for PDAC.
Keywords: Angiogenesis; Cancer-associated acinar-to-ductal metaplasia; Liver metastasis; Macrophages; Pancreatic cancer; Prognosis.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
The authors have no relevant financial or non-financial interests to disclose.
Figures

References
-
- Ansari D, Tingstedt B, Andersson B, Holmquist F, Sturesson C, Williamsson C, Sasor A, Borg D, Bauden M, Andersson R (2016) Pancreatic cancer: yesterday, today and tomorrow. Future Oncol 12:1929–1946. 10.2217/fon-2016-0010 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

