Amyloid-β targeting immunisation in aged non-human primate (Microcebus murinus)
- PMID: 36592872
- PMCID: PMC10023341
- DOI: 10.1016/j.bbi.2022.12.021
Amyloid-β targeting immunisation in aged non-human primate (Microcebus murinus)
Abstract
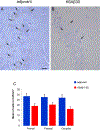
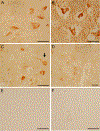
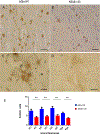
Non-human primates have an important translational value given their close phylogenetic relationship to humans. Studies in these animals remain essential for evaluating efficacy and safety of new therapeutic approaches, particularly in aging primates that display Alzheimer's disease (AD) -like pathology. With the objective to improve amyloid-β (Aβ) targeting immunotherapy, we investigated the safety and efficacy of an active immunisation with an Aβ derivative, K6Aβ1-30-NH2, in old non-human primates. Thirty-two aged (4-10 year-old) mouse lemurs were enrolled in the study, and received up to four subcutaneous injections of the vaccine in alum adjuvant or adjuvant alone. Even though antibody titres to Aβ were not high, pathological examination of the mouse lemur brains showed a significant reduction in intraneuronal Aβ that was associated with reduced microgliosis, and the vaccination did not lead to microhemorrhages. Moreover, a subtle cognitive improvement was observed in the vaccinated primates, which was probably linked to Aβ clearance. This Aβ derivative vaccine appeared to be safe as a prophylactic measure based on the brain analyses and because it did not appear to have detrimental effects on the general health of these old animals.
Keywords: Aging; Amyloid-β; Immunisation; Microcebus murinus; Non-human primate.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest EMS is an inventor on patents on Aβ immunotherapies that are assigned to New York University. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Alzforum 2022a. : https://www.alzforum.org/therapeutics
-
- Alzforum 2022b. :https://www.alzforum.org/news/research-news/finally-big-win-all-outcomes...).
-
- Asuni AA; Boutajangout A; Scholtzova H; Knudsen E; Li YS; Quartermain D; Frangione B; Wisniewski T; Sigurdsson EM Vaccination of Alzheimer’s Model Mice with Abeta Derivative in Alum Adjuvant Reduces Abeta Burden without Microhemorrhages. Eur J Neurosci 2006, 24 (9), 2530–2542. 10.1111/j.1460-9568.2006.05149.x. - DOI - PMC - PubMed
-
- Bastrup J; Hansen KH; Poulsen TBG, Kastaniegaard K; Asuni AA; Christensen S; Belling D; Helboe L; Stensballe A; Volbracht C. Anti-Aβ Antibody Aducanumab Regulates the Proteome of Senile Plaques and Closely Surrounding Tissue in a Transgenic Mouse Model of Alzheimer’s Disease. J Alzheimers Dis. 2021;79(1):249–265. doi: 10.3233/JAD-200715. PMID: 33252074. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

