Upcycling the anthracyclines: New mechanisms of action, toxicology, and pharmacology
- PMID: 36592899
- PMCID: PMC9840691
- DOI: 10.1016/j.taap.2022.116362
Upcycling the anthracyclines: New mechanisms of action, toxicology, and pharmacology
Abstract
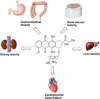
The anthracyclines are a family of natural products isolated from soil bacteria with over 2000 chemical representatives. Since their discovery seventy years ago by Waksman and co-workers, anthracyclines have become one of the best-characterized anticancer chemotherapies in clinical use. The anthracyclines exhibit broad-spectrum antineoplastic activity for the treatment of a variety of solid and liquid tumors, however, their clinical use is limited by their dose-limiting cardiotoxicity. In this review article, we discuss the toxicity of the anthracyclines on several organ systems, including new insights into doxorubicin-induced cardiotoxicity. In addition, we discuss new medicinal chemistry developments in the biosynthesis of new anthracycline analogs and the synthesis of new anthracycline analogs with diminished cardiotoxicity. Lastly, we review new studies that describe the repurposing of the anthracyclines, or "upcycling" of the anthracyclines, as anti-infective agents, or drugs for niche indications. Altogether, the anthracyclines remain a mainstay in the clinic with a potential new "lease on life" due to deeper insight into the mechanism underlying their cardiotoxicity and new developments into potential new clinical indications for their use. Keywords: Anthracycline, chemotherapy, toxicology, medicinal chemistry, biosynthesis.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Stephen Eric Nybo reports financial support was provided by National Science Foundation. Stephen Eric Nybo reports financial support was provided by National Institutes of Health.
Figures
References
-
- Acharya D, Miller I, Cui Y, Braun DR, Berres ME, Styles MJ, Li L, Kwan J, Rajski SR, Blackwell HE, Bugni TS, 2019. Omics Technologies to Understand Activation of a Biosynthetic Gene Cluster in Micromonospora sp. WMMB235: Deciphering Keyicin Biosynthesis. ACS Chem Biol 14, 1260–1270. 10.1021/acschembio.9b00223 - DOI - PMC - PubMed
-
- Adnani N, Chevrette MG, Adibhatla SN, Zhang F, Yu Q, Braun DR, Nelson J, Simpkins SW, McDonald BR, Myers CL, Piotrowski JS, Thompson CJ, Currie CR, Li L, Rajski SR, Bugni TS, 2017. Coculture of Marine Invertebrate-Associated Bacteria and Interdisciplinary Technologies Enable Biosynthesis and Discovery of a New Antibiotic, Keyicin. ACS Chem Biol acschembio.7b00688. 10.1021/acschembio.7b00688 - DOI - PMC - PubMed
-
- Arcamone F, Franceschi G, Orezzi P, Cassinelli G, Barbieri Wanda., Mondelli Rosanna., 1964. Daunomycin. I. The Structure of Daunomycinone. J Am Chem Soc 86, 5334–5335. 10.1021/ja01077a059 - DOI
-
- Bagre A, Patel PR, Jain K, Naqvi S, 2022. Emerging concerns of infectious diseases and drug delivery challenges. Nanotheranostics for Treatment and Diagnosis of Infectious Diseases 1–23. 10.1016/B978-0-323-91201-3.00013-X - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources