Diagnostic performance between in-house and commercial SARS-CoV-2 serological immunoassays including binding-specific antibody and surrogate virus neutralization test (sVNT)
- PMID: 36593231
- PMCID: PMC9806819
- DOI: 10.1038/s41598-022-26202-1
Diagnostic performance between in-house and commercial SARS-CoV-2 serological immunoassays including binding-specific antibody and surrogate virus neutralization test (sVNT)
Abstract
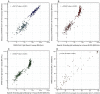
This study aimed to evaluate the correlation between in-house and commercial binding-specific IgG antibodies and between in-house and commercial SARS-CoV-2 surrogate virus neutralization tests (sVNT). Samples from healthcare workers who received vaccines against SARS-CoV-2 were tested for RBD-specific antibody, S-specific antibody, and in-house ELISA, commercial sVNT, and in-house sVNT, against wild-type SARS-CoV-2. Three hundred and five samples were included in the analysis. The correlation between S-specific binding antibodies and in-house ELISA was 0.96 (95% CI 0.96-0.97) and between RBD-specific antibodies and in-house ELISA was 0.96 (95% CI 0.95-0.97). The Cohen's kappa between in-house sVNT and the commercial test was 0.90 (95% CI 0.80, 1.00). If using 90% inhibition of sVNT as the reference standard, the optimal cut-off value of RBD-specific antibodies was 442.7 BAU/mL, the kappa, sensitivity, and specificity being 0.99, 99%, and 100%, respectively. The optimal cut-off value of S-specific antibodies was 1155.9 BAU/mL, the kappa, sensitivity, and specificity being 0.99, 100%, and 99%, respectively. This study demonstrated a very strong correlation between in-house ELISA and 2 commercial assays. There was also a very strong correlation between in-house and commercial SARS-CoV-2 sVNT, a finding of particular interest which will inform future research.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Kinetics and ability of binding antibody and surrogate virus neutralization tests to predict neutralizing antibodies against the SARS-CoV-2 Omicron variant following BNT162b2 booster administration.Clin Chem Lab Med. 2023 Apr 21;61(10):1875-1885. doi: 10.1515/cclm-2022-1258. Print 2023 Sep 26. Clin Chem Lab Med. 2023. PMID: 37078220
-
Evaluation of the correlation between the access SARS-CoV-2 IgM and IgG II antibody tests with the SARS-CoV-2 surrogate virus neutralization test.J Med Virol. 2022 Jan;94(1):335-341. doi: 10.1002/jmv.27338. Epub 2021 Oct 5. J Med Virol. 2022. PMID: 34524695 Free PMC article.
-
Evaluation of a biotin-based surrogate virus neutralization test for detecting postvaccination antibodies against SARS-CoV-2 variants in sera.Biochem Biophys Res Commun. 2023 Feb 26;646:8-18. doi: 10.1016/j.bbrc.2023.01.052. Epub 2023 Jan 19. Biochem Biophys Res Commun. 2023. PMID: 36696754 Free PMC article.
-
Can commercial automated immunoassays be utilized to predict neutralizing antibodies after SARS-CoV-2 infection? A comparative study between three different assays.Front Biosci (Landmark Ed). 2021 Jul 30;26(7):198-206. doi: 10.52586/4934. Front Biosci (Landmark Ed). 2021. PMID: 34340267
-
Evaluation of residual humoral immune response against SARS-CoV-2 by a surrogate virus neutralization test (sVNT) 9 months after BNT162b2 primary vaccination.J Infect Chemother. 2023 Jun;29(6):624-627. doi: 10.1016/j.jiac.2023.03.002. Epub 2023 Mar 11. J Infect Chemother. 2023. PMID: 36914095 Free PMC article.
Cited by
-
Safety and immunogenicity of the third and fourth doses of vaccine against SARS-CoV-2 following a 2-dose regimen of inactivated whole-virion SARS-CoV-2 vaccine.Sci Rep. 2023 Nov 13;13(1):19736. doi: 10.1038/s41598-023-45735-7. Sci Rep. 2023. PMID: 37957189 Free PMC article.
-
A preliminary study on the effectiveness of maternal to neonatal transfer of antibodies against SARS-CoV-2 in the women vaccinated with heterologous CoronaVac-ChAdOx1.Hum Vaccin Immunother. 2023 Dec 31;19(1):2196912. doi: 10.1080/21645515.2023.2196912. Epub 2023 Apr 14. Hum Vaccin Immunother. 2023. PMID: 37057690 Free PMC article.
-
TIRTL-seq: Deep, quantitative, and affordable paired TCR repertoire sequencing.bioRxiv [Preprint]. 2024 Oct 31:2024.09.16.613345. doi: 10.1101/2024.09.16.613345. bioRxiv. 2024. PMID: 39345544 Free PMC article. Preprint.
-
Robust immune response to COVID-19 vaccination in the island population of Greenland.Commun Med (Lond). 2024 Sep 6;4(1):173. doi: 10.1038/s43856-024-00602-y. Commun Med (Lond). 2024. PMID: 39242878 Free PMC article.
-
Safety and immunogenicity of CoronaVac and ChAdOx1 heterologous prime-boost vaccines in an overweight population in Chiang Mai, Thailand.Vaccine X. 2024 Mar 16;18:100475. doi: 10.1016/j.jvacx.2024.100475. eCollection 2024 Jun. Vaccine X. 2024. PMID: 38549951 Free PMC article.
References
-
- Zimmer, C., Corum, J. & Wee, S. L. Coronavirus vaccine tracker, vol. 2022 (2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

