Evolution of CRISPR-associated endonucleases as inferred from resurrected proteins
- PMID: 36593295
- PMCID: PMC10324499
- DOI: 10.1038/s41564-022-01265-y
Evolution of CRISPR-associated endonucleases as inferred from resurrected proteins
Abstract
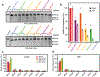
Clustered regularly interspaced short palindromic repeats (CRISPR)-associated Cas9 is an effector protein that targets invading DNA and plays a major role in the prokaryotic adaptive immune system. Although Streptococcus pyogenes CRISPR-Cas9 has been widely studied and repurposed for applications including genome editing, its origin and evolution are poorly understood. Here, we investigate the evolution of Cas9 from resurrected ancient nucleases (anCas) in extinct firmicutes species that last lived 2.6 billion years before the present. We demonstrate that these ancient forms were much more flexible in their guide RNA and protospacer-adjacent motif requirements compared with modern-day Cas9 enzymes. Furthermore, anCas portrays a gradual palaeoenzymatic adaptation from nickase to double-strand break activity, exhibits high levels of activity with both single-stranded DNA and single-stranded RNA targets and is capable of editing activity in human cells. Prediction and characterization of anCas with a resurrected protein approach uncovers an evolutionary trajectory leading to functionally flexible ancient enzymes.
© 2023. The Author(s), under exclusive licence to Springer Nature Limited.
Figures












References
-
- Mohanraju P et al. Alternative functions of CRISPR–Cas systems in the evolutionary arms race. Nature Reviews Microbiology (2022). - PubMed
-
- Garneau JE et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468, 67–71 (2010). - PubMed
-
- Barrangou R et al. CRISPR Provides Acquired Resistance Against Viruses in Prokaryotes. Science 315, 1709–1712 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

