Quantum view of Li-ion high mobility at carbon-coated cathode interfaces
- PMID: 36594017
- PMCID: PMC9803833
- DOI: 10.1016/j.isci.2022.105794
Quantum view of Li-ion high mobility at carbon-coated cathode interfaces
Abstract
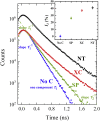
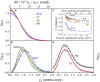
Lithium-ion batteries (LIBs) are among the most promising power sources for electric vehicles, portable electronics and smart grids. In LIBs, the cathode is a major bottleneck, with a particular reference to its low electrical conductivity and Li-ion diffusivity. The coating with carbon layers is generally employed to enhance the electrical conductivity and to protect the active material from degradation during operation. Here, we demonstrate that this layer has a primary role in the lithium diffusivity into the cathode nanoparticles. Positron is a useful quantum probe at the electroactive materials/carbon interface to sense the mobility of Li-ion. Broadband electrical spectroscopy demonstrates that only a small number of Li-ions are moving, and that their diffusion strongly depends on the type of carbon additive. Positron annihilation and broadband electrical spectroscopies are crucial complementary tools to investigate the electronic effect of the carbon phase on the cathode performance and Li-ion dynamics in electroactive materials.
Keywords: Electrochemical energy storage; Electrochemical materials science; Electrochemistry; Materials application; Materials science.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Pagot G., Bandiera M., Vezzù K., Migliori A., Bertoncello R., Negro E., Morandi V., Di Noto V. High valence transition metal-doped olivine cathodes for superior energy and fast cycling lithium batteries. J. Mater. Chem. 2020;8:25727–25738. doi: 10.1039/d0ta06865a. - DOI
-
- Pagot G., Bertasi F., Nawn G., Negro E., Carraro G., Barreca D., Maccato C., Polizzi S., Di Noto V. High-performance olivine for lithium batteries: effects of Ni/Co doping on the properties of LiFeαNiβCoγPO4 cathodes. Adv. Funct. Mater. 2015;25:4032–4037. doi: 10.1002/adfm.201501167. - DOI
LinkOut - more resources
Full Text Sources

