Cargo selection in endoplasmic reticulum-to-Golgi transport and relevant diseases
- PMID: 36594468
- PMCID: PMC9797344
- DOI: 10.1172/JCI163838
Cargo selection in endoplasmic reticulum-to-Golgi transport and relevant diseases
Abstract
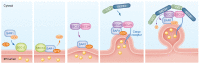
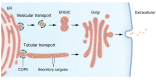
Most proteins destined for the extracellular space or various intracellular compartments must traverse the intracellular secretory pathway. The first step is the recruitment and transport of cargoes from the endoplasmic reticulum (ER) lumen to the Golgi apparatus by coat protein complex II (COPII), consisting of five core proteins. Additional ER transmembrane proteins that aid cargo recruitment are referred to as cargo receptors. Gene duplication events have resulted in multiple COPII paralogs present in the mammalian genome. Here, we review the functions of each COPII protein, human disorders associated with each paralog, and evidence for functional conservation between paralogs. We also provide a summary of current knowledge regarding two prototypical cargo receptors in mammals, LMAN1 and SURF4, and their roles in human health and disease.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

