Neurodegeneration in a novel invertebrate model system: Failed microtubule-mediated cell adhesion and unraveling of macroglia
- PMID: 36594894
- PMCID: PMC10037207
- DOI: 10.1002/cne.25450
Neurodegeneration in a novel invertebrate model system: Failed microtubule-mediated cell adhesion and unraveling of macroglia
Abstract
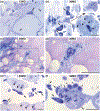
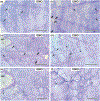
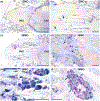
Neurodegenerative diseases are among the main causes of death in the United States, leading to irreversible disintegration of neurons. Despite intense international research efforts, cellular mechanisms that initiate neurodegeneration remain elusive, thus inhibiting the development of effective preventative and early onset medical treatment. To identify underlying cellular mechanisms that initiate neuron degeneration, it is critical to identify histological and cellular hallmarks that can be linked to underlying biochemical processes. Due to the poor tissue preservation of degenerating mammalian brain tissue, our knowledge regarding histopathological hallmarks of early to late degenerative stages is only fragmentary. Here, we introduce a novel model organism to study histological hallmarks of neurodegeneration, the spider Cupiennius salei. We utilized toluidine blue-stained 0.9-μm serial semithin and 50-nm ultrathin sections of young and old spider nervous tissue. Our findings suggest that the initial stages of neurodegeneration in spiders may be triggered by (1) dissociation of neuron- and glia-derived microtubules, and (2) the weakening of microtubule-associated desmosomal junctions that lead to the unraveling of neuron-insulating macroglia, compromising the structural integrity of affected neurons. The involvement of macroglia in the disposal of neuronal debris described here-although different in the proposed transport mechanisms-shows resemblance to the mammalian glymphatic system. We propose that this model system is highly suitable to investigate invertebrate neurodegenerative processes from early onset to scar formation and that this knowledge may be useful for the study of neurodegeneration in mammalian tissue.
Keywords: Cupiennius salei; cell adhesion; desmosomes; macroglia; microtubules.
© 2023 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no competing financial interests.
Figures











References
-
- Babu KS, & Barth FG (1984). Neuroanatomy of the central nervous system of the wandering spider, Cupiennius salei (Arachnida, Araneida). Zoomorphology, 104(6), 344–359. doi:10.1007/BF00312185 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

