Mapping hematologists' HIV testing behavior among lymphoma patients-A mixed-methods study
- PMID: 36595516
- PMCID: PMC9810165
- DOI: 10.1371/journal.pone.0279958
Mapping hematologists' HIV testing behavior among lymphoma patients-A mixed-methods study
Abstract
Background: HIV testing among patients with malignant lymphoma (PWML) is variably implemented. We evaluated HIV testing among PWML, and mapped factors influencing hematologists' testing behavior.
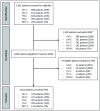
Materials: We conducted a mixed-methods study assessing HIV testing among PWML, factors influencing HIV testing and opportunities for improvement in five hospitals in the region of Amsterdam, the Netherlands. The proportion of PWML tested for HIV within 3 months before or after lymphoma diagnosis and percentage positive were assessed from January 2015 through June 2020. Questionnaires on intention, behavior and psychosocial determinants for HIV testing were conducted among hematologists. Through twelve semi-structured interviews among hematologists and authors of hematology guidelines, we further explored influencing factors and opportunities for improvement.
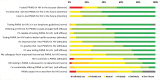
Findings: Overall, 1,612 PWML were included for analysis, including 976 patients newly diagnosed and 636 patients who were referred or with progressive/relapsed lymphoma. Seventy percent (678/976) of patients newly diagnosed and 54% (343/636) of patients with known lymphoma were tested for HIV. Overall, 7/1,021 (0.7%) PWML tested HIV positive, exceeding the 0.1% cost-effectiveness threshold. Questionnaires were completed by 40/77 invited hematologists, and 85% reported intention to test PWML for HIV. In the interviews, hematologists reported varying HIV testing strategies, including testing all PWML or only when lymphoma treatment is required. Recommendations for improved HIV testing included guideline adaptations, providing electronic reminders and monitoring and increasing awareness.
Conclusions: Missed opportunities for HIV testing among PWML occurred and HIV test strategies varied among hematologists. Efforts to improve HIV testing among PWML should include a combination of approaches.
Copyright: © 2023 Bogers et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Dr. Bogers has nothing to disclose. Dr. Zimmermann has nothing to disclose. Dr. Ndong has nothing to disclose. Dr. Davidovich has nothing to disclose. Dr. Kersten reports consulting fees: Kite/Gilead; BMS/Celgene; Novartis; Miltenyi Biotec; Adicet Bio: to institution and payment for honoraria: Kite/Gilead; Roche; BMS/Celgene: to institution and participation on a Data Safety Monitoring Board or Advisory Board: SUBITO study (high dose chemotherapy in breast cancer): No financial compensation. Dr. Reiss reports grants or contracts: Gilead Sciences; ViiV Healthcare; Merck: Investigator-initiated study grants to institution; not related to current work and Participation on a Data Safety Monitoring Board or Advisory Board: Gilead Sciences; ViiV Healthcare; Merck: Honoraria for scientific advisory board participation paid to institution. Dr. Schim van der Loeff has nothing to disclose. Dr. Geerlings has nothing to disclose. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- World Health Organization. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. Geneva 2021. Available online at https://www.who.int/publications/i/item/9789240027077. Accessed 5 December 2022.
-
- Sullivan AK, Raben D, Reekie J, Rayment M, Mocroft A, Esser S, et al.. Feasibility and effectiveness of indicator condition-guided testing for HIV: results from HIDES I (HIV indicator diseases across Europe study). PLoS One. 2013;8(1):e52845. Epub 2013/01/24. doi: 10.1371/journal.pone.0052845 ; PubMed Central PMCID: PMC3546115. - DOI - PMC - PubMed
-
- Raben D, Sullivan AK, Mocroft A, Kutsyna G, Hadziosmanovic V, Vassilenko A, et al.. Improving the evidence for indicator condition guided HIV testing in Europe: Results from the HIDES II Study—2012–2015. PLoS One. 2019;14(8):e0220108. Epub 2019/08/14. doi: 10.1371/journal.pone.0220108 ; PubMed Central PMCID: PMC6692030. - DOI - PMC - PubMed
-
- Omland LH, Legarth R, Ahlstrom MG, Sorensen HT, Obel N. Five-year risk of HIV diagnosis subsequent to 147 hospital-based indicator diseases: a Danish nationwide population-based cohort study. Clin Epidemiol. 2016;8:333–40. Epub 2016/09/24. doi: 10.2147/CLEP.S101288 ; PubMed Central PMCID: PMC5019186. - DOI - PMC - PubMed
-
- Scognamiglio P, Chiaradia G, De Carli G, Giuliani M, Mastroianni CM, Aviani Barbacci S, et al.. The potential impact of routine testing of individuals with HIV indicator diseases in order to prevent late HIV diagnosis. BMC Infect Dis. 2013;13:473. Epub 2013/10/12. doi: 10.1186/1471-2334-13-473 ; PubMed Central PMCID: PMC3852490. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

