Adverse drug event detection using natural language processing: A scoping review of supervised learning methods
- PMID: 36595517
- PMCID: PMC9810201
- DOI: 10.1371/journal.pone.0279842
Adverse drug event detection using natural language processing: A scoping review of supervised learning methods
Abstract
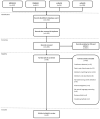
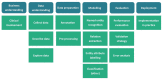
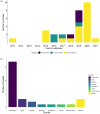
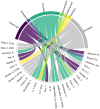
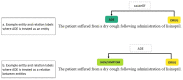
To reduce adverse drug events (ADEs), hospitals need a system to support them in monitoring ADE occurrence routinely, rapidly, and at scale. Natural language processing (NLP), a computerized approach to analyze text data, has shown promising results for the purpose of ADE detection in the context of pharmacovigilance. However, a detailed qualitative assessment and critical appraisal of NLP methods for ADE detection in the context of ADE monitoring in hospitals is lacking. Therefore, we have conducted a scoping review to close this knowledge gap, and to provide directions for future research and practice. We included articles where NLP was applied to detect ADEs in clinical narratives within electronic health records of inpatients. Quantitative and qualitative data items relating to NLP methods were extracted and critically appraised. Out of 1,065 articles screened for eligibility, 29 articles met the inclusion criteria. Most frequent tasks included named entity recognition (n = 17; 58.6%) and relation extraction/classification (n = 15; 51.7%). Clinical involvement was reported in nine studies (31%). Multiple NLP modelling approaches seem suitable, with Long Short Term Memory and Conditional Random Field methods most commonly used. Although reported overall performance of the systems was high, it provides an inflated impression given a steep drop in performance when predicting the ADE entity or ADE relation class. When annotating corpora, treating an ADE as a relation between a drug and non-drug entity seems the best practice. Future research should focus on semi-automated methods to reduce the manual annotation effort, and examine implementation of the NLP methods in practice.
Copyright: © 2023 Murphy et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Laatikainen O., Miettunen J., Sneck S., Lehtiniemi H., Tenhunen O., & Turpeinen M. (2017). The prevalence of medication-related adverse events in inpatients—a systematic review and meta-analysis. European Journal of Clinical Pharmacology, 73(12), 1539–1549. doi: 10.1007/s00228-017-2330-3 - DOI - PubMed
-
- Jha A. K., Kuperman G. J., Teich J. M., Leape L., Shea B., Rittenberg E., et al.. (1998). Identifying Adverse Drug Events: Development of a Computer-based Monitor and Comparison with Chart Review and Stimulated Voluntary Report. Journal of the American Medical Informatics Association, 5(3), 305–314. doi: 10.1136/jamia.1998.0050305 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

