Neutral vs. non-neutral genetic footprints of Plasmodium falciparum multiclonal infections
- PMID: 36595546
- PMCID: PMC9838855
- DOI: 10.1371/journal.pcbi.1010816
Neutral vs. non-neutral genetic footprints of Plasmodium falciparum multiclonal infections
Abstract
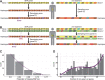
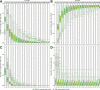
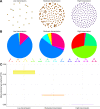
At a time when effective tools for monitoring malaria control and eradication efforts are crucial, the increasing availability of molecular data motivates their application to epidemiology. The multiplicity of infection (MOI), defined as the number of genetically distinct parasite strains co-infecting a host, is one key epidemiological parameter for evaluating malaria interventions. Estimating MOI remains a challenge for high-transmission settings where individuals typically carry multiple co-occurring infections. Several quantitative approaches have been developed to estimate MOI, including two cost-effective ones relying on molecular data: i) THE REAL McCOIL method is based on putatively neutral single nucleotide polymorphism loci, and ii) the varcoding method is a fingerprinting approach that relies on the diversity and limited repertoire overlap of the var multigene family encoding the major Plasmodium falciparum blood-stage antigen PfEMP1 and is therefore under selection. In this study, we assess the robustness of the MOI estimates generated with these two approaches by simulating P. falciparum malaria dynamics under three transmission conditions using an extension of a previously developed stochastic agent-based model. We demonstrate that these approaches are complementary and best considered across distinct transmission intensities. While varcoding can underestimate MOI, it allows robust estimation, especially under high transmission where repertoire overlap is extremely limited from frequency-dependent selection. In contrast, THE REAL McCOIL often considerably overestimates MOI, but still provides reasonable estimates for low and moderate transmission. Regardless of transmission intensity, results for THE REAL McCOIL indicate that an inaccurate tail at high MOI values is generated, and that at high transmission, an apparently reasonable estimated MOI distribution can arise from some degree of compensation between overestimation and underestimation. As many countries pursue malaria elimination targets, defining the most suitable approach to estimate MOI based on sample size and local transmission intensity is highly recommended for monitoring the impact of intervention programs.
Copyright: © 2023 Labbé et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- World Health Organization. World malaria report 2021. World Health Organization; 2021.
-
- Kolakovich KA, Ssengoba A, Wojcik K, Tsuboi T, Al-Yaman F, Alpers M, et al.. Plasmodium vivax:favored gene frequencies of the merozoite surface protein-1 and the multiplicity of infection in a malaria endemic region. Experimental Parasitology. 1996;83: 11–18. doi: 10.1006/expr.1996.0044 - DOI - PubMed
-
- Konaté L, Zwetyenga J, Rogier C, Bischoff E, Fontenille D, Tall A, et al.. 5. Variation of Plasmodium falciparum msp1 block 2 and msp2 allele prevalence and of infection complexity in two neighbouring Senegalese villages with different transmission conditions. Transactions of The Royal Society of Tropical Medicine and Hygiene. 1999;93: 21–28. doi: 10.1016/S0035-9203(99)90323-1 - DOI - PubMed
-
- Anderson TJC, Haubold B, Williams JT, Estrada-Franco§ JG, Richardson L, Mollinedo R, et al.. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Molecular Biology and Evolution. 2000;17: 1467–1482. doi: 10.1093/oxfordjournals.molbev.a026247 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

